Bond dipole and molecular dipole are two words used to describe the chemical and physical characteristics of chemical species. The primary distinction between bond dipole and molecular dipole is that bond dipole refers to the formation of two polar ends in a specific chemical bond, whereas molecular dipole refers to the formation of two polar ends in a specific chemical molecule.
Interesting Science Videos
What is Molecular Dipole?
A molecular dipole is the existence of two oppositely charged ends in the same molecule. The net polarity of the molecule causes this form of dipole moment. The net polarity, or overall polarity, of a molecule is determined by the shape of the molecule and the polarity of each chemical connection. A molecule with polar chemical bonds may not have a net polarity due to the shape that balances the net polarity to zero. Consider a linear molecule with two polar bonds. In this case, the dipole moment vectors of the two bonds cancel out. For example, carbon dioxide.
Understanding Molecular Dipole with Structures
When a molecule possesses a dipole, it is referred to as a polar molecule. There are two key criteria for a molecule to have a dipole. The molecule must first have some polar connections. Second, the dipoles generated by these bonds must not cancel out as a result of the molecule’s symmetry. This is one of our primary concerns in molecular form. We’d like to know if certain areas of the molecule have a greater electron density than others. This will have a significant impact on both how the molecule interacts with other molecules and its chemistry.
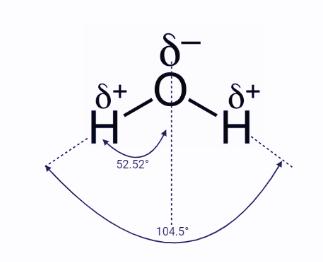
Let’s look at a few major scenarios to illustrate this point. Water is one of the most significant chemical compounds on earth. Water’s characteristics are influenced by the way electrons are dispersed in the molecule in a variety of ways. Water is made up of an oxygen atom in the center that is covalently bound to two hydrogen atoms. We may derive from the VSEPR model that water has a bent structure. To determine if water is polar, we must study the bonds inside the molecule. Because oxygen is substantially more electronegative than hydrogen, we would define the O-H bonds in water as polar covalent bonds.
This means that each of these bonds has a dipole formed by the partial positive charge on hydrogen and the partial negative charge on oxygen. To determine if the entire molecule has a dipole, we must examine the geometrical arrangement of these dipoles. Because water is bent, when the dipoles of the bonds are added together, we discover a net dipole. That is, water has a positive (where the hydrogens are) and a negative (where the oxygens are). Because each “side” containing a hydrogen is symmetric, the dipole is symmetric in the “middle” of the molecule. This is most clearly observed in the water dipole graphic. The net (or molecular dipole) is a type of molecular dipole.
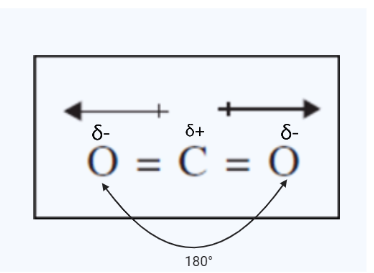
Carbon dioxide, on the other hand, although having polar covalent connections, does not have a molecule dipole. This is due to the molecule’s shape. The oxygens in the molecules have a higher electron density and consequently a partial negative charge. As a result, the carbon-oxygen double bonds become polar. However, because the molecule is linear, the two dipoles from the two bonds in the molecule point in completely opposite directions. As a result, the dipoles cancel and the molecule has no net dipole.
References
- https://www.differencebetween.com/difference-between-bond-dipole-and-molecular-dipole/
- https://wisc.pb.unizin.org/chem109fall2021ver02/chapter/dipole-dipole-attractions/
- https://www.sciencedirect.com/topics/physics-and-astronomy/molecular-dipole-moment
- https://ch301.cm.utexas.edu/section2.php?target=imfs/vsepr/shape-dipole.html
