
Elements with properties that are somewhere between those of metals and non-metals are referred to as metalloids. These substances, which are situated in the middle of the periodic table and are also referred to as semi-metals, are capable of performing either the role of an insulator or a conductor. Due to the diverse range of applications and characteristics that they offer, they are vital elements in a variety of different sectors.
Boron, germanium, silicon, antimony, arsenic, and tellurium are the six most common metalloids. In addition to the aforementioned six elements, the classification of metalloid elements occasionally encompasses the inclusion of bismuth, polonium, and astatine. The presence of ambiguity can be primarily attributed to a lack of well-defined properties that are universally recognized as characteristic of all metalloids.
Despite of the metallic look, metalloids are typically brittle and weak electrical conductors. These substances are often chemically distinct from metals. Most of their physical and chemical characteristics fall somewhere in the middle. The periodic table exhibits a distinct region known as the metalloid zone, which serves as the boundary between elements displaying prominent metallic characteristics and those exhibiting distinct nonmetallic properties.
What is a metalloid?
The term “metalloids” (also known as “semimetals”) is used to describe elements that exhibit properties that lie between those typically associated with metals and nonmetals. The precise measure of elements that are classified as metalloids is a subject of ongoing debate; however, there is generally agreed upon that a minimum of six elements fall within this category.
Metalloids generally exhibit metallic luster, yet they tend to possess brittleness and display moderate electrical conductivity. From a chemical properties perspective, it is commonly observed that these elements tend to exhibit non-metallic characteristics. Metalloids possess the capacity to engage in the formation of metallic alloys. The metalloid elements typically exhibit physical and chemical properties that are predominantly of an intermediate nature. Generally speaking, these elements exhibit a high degree of fragility, which consequently limits their potential for utilization in structural contexts. Metalloids and their compounds find applications in a variety of fields including alloys, catalysts, biological agents, glasses, flame retardants, optical storage and optoelectronics, semiconductors, pyrotechnics, and electronics.
Position of metalloids in the periodic table
As stated earlier, metalloids are a category of chemical elements that are positioned in an oblique arrangement between the metallic and nonmetallic elements on the periodic table. The series of metalloid elements extends from Group 13 to either Group 16, 17, or 18, depending on the inclusion of certain elements within the metalloid classification. The elements classified as metals are situated to the left of this line of metalloid elements, while the elements classified as non-metals are situated to their right. The sole anomaly to this principle is the chemical element hydrogen, which is categorized as a nonmetal despite its placement on the left-hand side of the periodic table.
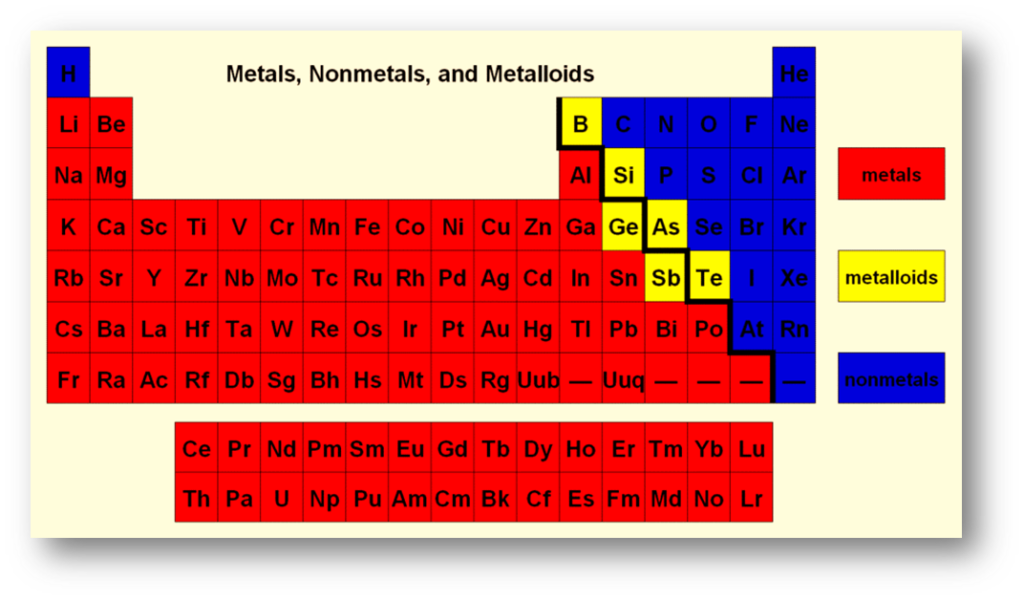
The diagonal arrangement of metalloids represents a deviation from the general trend where elements with similar properties are typically grouped together vertically. The presence of diagonal similarities between certain elements and their lower right neighbors, such as lithium and magnesium, beryllium and aluminum, and boron and silicon, indicates the existence of a corresponding effect. Rayner-Canham has observed that similar patterns can be found in the carbon-phosphorus, nitrogen-sulfur, and three additional d-block series.
Metalloids elements
The number of elements classified as metalloids varies between six and nine, depending on the specific definition employed by scientists.
Generally agreed upon as metalloids are the following six elements:
- Boron possesses an atomic number of 5. It is utilized in various chemical compounds across multiple applications.
- Pure crystalline boron exhibits a black, lustrous appearance and possesses exceptional hardness.
- Boric acid and borates exhibit a favorable safety profile in relation to animals while demonstrating toxicity towards arthropods. However, it is important to note that these substances are vital for promoting optimal plant growth.
- It is commonly employed as an additive in the process of enhancing the hardness of steel and glass materials.
- Boron-containing compounds find applications as insecticides and fertilizers.
- Silicon possesses an atomic number of 14.
- This metalloid exhibits a remarkable degree of versatility, finding widespread utilization across various domains, with its primary applications being in the fields of semiconductors and construction.
- Silicon in its elemental form exhibits significant reactivity, and its various compounds are frequently encountered in geological formations such as sands, rocks, and soils.
- The material exhibits low electrical conductivity, which demonstrates an enhanced efficiency as the temperature increases.
- This substance finds application in the production of alloys, glass, enamels, and various other ceramics.
- Germanium, with an atomic number of 32, is frequently employed as a semiconductor material in the construction of transistors.
- Exhibits low electrical conductivity that demonstrates enhanced efficiency at higher temperatures.
- The material exhibits a rigid and fragile nature, characterized by a metallic visual aspect.
- It is utilized as an additive to enhance the corrosion resistance of specific alloys.
- Semiconductors and infrared detectors frequently employ this particular material.
- Arsenic possesses an atomic number 33.
- The interference with cellular respiration, a fundamental process in which cells generate energy, presents a significant threat to human health.
- The element possesses the capacity to establish a maximum of three covalent bonds, thereby facilitating its ability to readily form bonds with numerous metallic elements.
- In its elemental state, arsenic is chemically unreactive, yet it exhibits high toxicity towards both animals and plants when it undergoes a transformation into arsine or other organic derivatives.
- It can serve as an additive for the purpose of enhancing the hardness of lead and various other metal alloys.
- It is employed as an active ingredient in certain herbicides and insecticides, as well as serving as a wood preservative.
- Tellurium, with an atomic number of 52, is frequently employed as an alloying agent.
- It is crystalline in nature
- It is a highly uncommon occurrence and can be discovered within extracted mineral deposits.
- The substance exhibits stability when exposed to water, while it undergoes dissolution when subjected to nitric acid.
- It is frequently employed as an additive to enhance the mechanical strength and resistance to corrosion in specific alloys.
- Antimony possesses an atomic number of 51. It is frequently employed in the formulation of alloys and the production of paints.
- Antimony is characterized by an atomic number of 51. The utilization of this substance is commonly observed in the composition of alloys and the manufacturing of paints.
- Exhibits a lustrous, silver-white metallic appearance.
- This substance finds application in the production of alloys, glass, enamels, and various other ceramic materials.
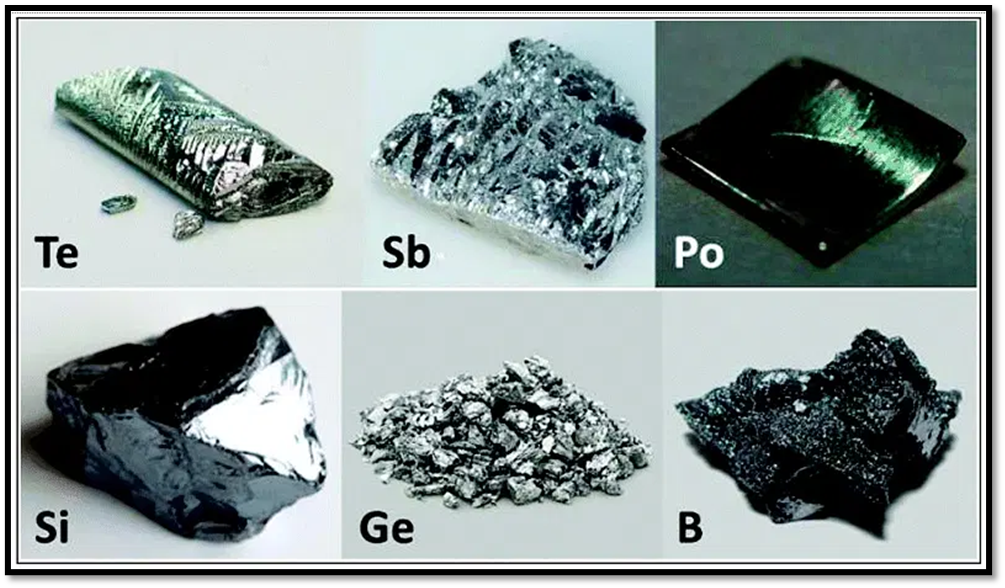
Figure source: https://www.chemistrylearner.com/metalloids
There are three more elements that are classified as metalloids by certain researchers. There is a lack of agreement on whether these elements should be considered metals or nonmetals.
- Polonium, possessing an atomic number of 84, exhibits exceptional radioactivity and is characterized by its scarcity within the Earth’s crust.
- This substance exhibits a high level of radioactivity and releases alpha particles.
- The purpose of this device is to eliminate static electricity in machinery or remove dust particles from photographic film.
- Thermoelectric power in space satellites often utilizes lightweight heat sources.
- Its atomic number is 85
- The radioactive element currently lacks practical applications beyond the boundaries of the research laboratory.
- The atomic number of bismuth is 83.
- This substance is employed for the purpose of extinguishing fires.
Properties of metalloids
Metalloids typically exhibit the physical characteristics associated with metals. These materials exhibit characteristics of hardness and brittleness and demonstrate properties akin to those of semiconductors. Metalloids also exhibit chemical properties similar to those of nonmetals. Therefore, metalloids are generally not employed in mechanical applications. Nonetheless, the manifestation of this conduct is contingent upon the specific constituents with which they are engaging in a reaction. In certain chemical reactions, metalloids exhibit nonmetallic behavior, while in others, they exhibit metallic behavior.
Physical properties of metalloids
The physical properties of metalloids exhibit a combination of characteristics found in both metals and nonmetals. The following are a few examples of physical properties associated with metalloids:
- Electrical conductivity: Metalloids exhibit lower electrical conductivity compared to metals. Indeed, numerous metalloids exhibit the characteristics of semiconductors. This implies that their electrical conductivity can vary based on the concentration of impurities or the influence of temperature.
- Thermal conductivity: Metalloids exhibit higher thermal conductivity compared to nonmetals, yet lower than that of metals.
- Mechanical properties: Metalloids exhibit limited ductility, rendering them highly susceptible to brittleness. Metalloids, due to their inherent properties, are not suitable for utilization in structural applications.
- Hardness: Metalloids exhibit a diverse spectrum of hardness levels. As an example, it can be observed that arsenic possesses a Mohs hardness value of 3.5, whereas boron exhibits a significantly higher Mohs hardness value of 9.3. In terms of comparison, it is noteworthy that gold possesses a Mohs hardness rating of 2.5, while diamond exhibits a significantly higher Mohs hardness rating of 10.
- Metallic appearance: The majority of metalloids exhibit a visual characteristic characterized by a lustrous and reflective surface, identical to the appearance commonly associated with various metallic elements.
- Density: The densities of metalloids exhibit significant variation. For example, the density of silicon is recorded as 2.33 g/cm3, whereas antimony exhibits a density of 6.69 g/cm3.
- Shape: Metalloids exhibit a solid state at standard room temperature.
Chemical properties of metalloids
Metalloids typically exhibit chemical properties similar to those of nonmetals. The following are several prevalent chemical properties associated with metalloids:
- Reactivity: Metalloids exhibit a tendency to participate in covalent bonding, similar to that observed in nonmetals. Monatomic anions are not typically formed by these elements, exhibiting a characteristic behavior commonly observed in metals.
- Covalent bonds: Metalloids exhibit a tendency to engage in covalent bonding, unlike metals which have the ability to form monatomic ions.
- Electronegativity: Electronegativity relates to the tendency of an atom to attract other elements during the process of chemical bonding. The magnitude of the attraction increases with higher numerical values. Metalloids generally exhibit electronegativity values ranging from 1.8 to 2.2.
- Formation of alloy: The incorporation of metalloids into metallic structures can result in the formation of alloys. A typical example involves the combination of antimony and lead, resulting in the creation of antimonial lead alloys, which find application in the field of ammunition.
Metalloids use
Metalloids possess a diverse array of applications owing to their distinctive characteristics.
- Boron is commonly employed in nuclear reactors owing to its notable capacity for neutron absorption. Additionally, it finds application in the production of alloys, such as boron steel, which exhibits enhanced strength compared to conventional steel while maintaining a lightweight nature.
- Silicon finds extensive applications in various technological domains, including semiconductor chips, solar cells, and microprocessors. Additionally, it plays a crucial role in the manufacturing process of glass fibers, which are indispensable components for the creation of optical cables utilized in telecommunications networks.
- Germanium possesses significant applications in the field of electronics owing to its remarkable signal amplification capabilities. Additionally, it finds utility as an alloying agent in copper alloys, as it effectively enhances strength while simultaneously reducing electrical resistivity.
- Arsenic finds its primary application in the production of pesticides. Additionally, it is employed as a hardening agent in lead alloys and in thermoelectric devices, owing to its low thermal expansion coefficient, which renders it advantageous for temperature regulation purposes.
- Antimony is frequently encountered in the form of an alloy with lead due to its ability to enhance strength and durability. This particular characteristic renders it well-suited for applications in metal bearings that are subjected to elevated temperatures or experience significant friction.
Application of metalloids
The semiconducting qualities of metalloids make them useful in many different contexts as discussed below:
- Semiconductors: Silicon is extensively utilized as a semiconductor material and is present in the majority of electronic devices. Furthermore, it should be noted that germanium doped with arsenic exhibits semiconducting properties that are of significant commercial value. The high charge mobility exhibited by antimony renders it advantageous for certain specialized semiconductor applications.
- Biological agents: The six elements are widely acknowledged as metalloids, possessing dietary, medicinal, or toxic attributes. Antimony and arsenic compounds exhibit significant toxicity, while silicon, boron, and potentially arsenic are considered crucial trace elements. Silicon, boron, antimony, and arsenic exhibit potential medical applications. Simultaneously, tellurium and germanium are believed to possess promising qualities.
- Flame retardants: Flame retardants comprising silicon, boron, antimony, and arsenic compounds have been employed in various applications. Boron, in the form of borax, has been utilized as a textile flame retardant since at least the 18th century. Silicon compounds, such as silanes, silsesquioxane, silicones, silicates, and silica, have been developed as potential substitutes for halogenated products due to their improved flame retardancy properties when incorporated into plastic materials.
- Silicon rubber: The process of polymerization involves the combination of silicon and oxygen to produce a polymer that possesses a backbone consisting of silicone and oxygen atoms. In this polymer, the branches are commonly composed of methyl groups. Modifying the polymer chain allows for the attainment of a diverse array of properties. Silicone is extensively employed as a sealant, lubricant, insulating material, and in the fabrication of cooking utensils.
- Formation of glass: The oxides SiO2, B2O3, Sb2O3, and As2O3 readily make glasses. Also, TeO2 makes a glass, but this needs the addition of an impurity or a “heroic quench rate”; otherwise, it results in the crystalline form. These compounds can be used in domestic, industrial, and chemical glassware and optics. On the other side, boron trioxide can be used as a glass fibre additive and also as a borosilicate glass component, which can be widely used for domestic ovenware and laboratory glassware for its low thermal expansion.
- Alloy: Metalloids are frequently employed as constituents in metal alloys. Illustrative instances encompass the incorporation of silicon within aluminum and the infusion of boron into iron. Additional metalloids that can be used for alloying purposes are antimony and tellurium.
- Catalyst: Trichloride and boron trifluoride have been identified as viable catalysts for applications in the fields of electronics and organic synthesis. Additionally, the utilization of tribromide has been observed in the manufacturing process of diborane. Moreover, the substitution of toxic phosphorus ligands with non-toxic boron ligands has been observed in select transition metal catalysts. Silica sulfuric acid (SiO2OSO3H) possesses utility in various organic reactions. Germanium dioxide is occasionally utilized as a catalyst in the production of PET plastic containers. However, less expensive antimony compounds, specifically triacetate or trioxide, are more frequently employed for a similar catalytic function. It is worth noting that the use of antimony compounds raises concerns regarding potential contamination of beverages and food products.
- Medical application: All six elements commonly referred to as metalloids are recognized for their inherent toxicity, as well as their potential medicinal and nutritional attributes. Compounds consisting of antimony and arsenic have been widely recognized for their notably high toxicity. Nevertheless, boron, arsenic, and silicon hold significant importance as trace elements. Boron, arsenic, silicon, and antimony are recognized for their diverse applications in the field of medicine. Germanium and tellurium, the two remaining elements, exhibit significant potential for medicinal applications.
- Moreover, boron finds application in the formulation of herbicides and insecticides. This particular element is classified as an active trace element, possessing various properties such as antiseptic, antiviral, and antifungal effects when present in the form of boric acid.
Conclusion
Thus, presented herein is a comprehensive summary of metalloids. Metalloids exhibit remarkable versatility due to their distinctive properties, rendering them highly advantageous for a diverse array of applications encompassing semiconductors, solar cells, lead alloys, thermoelectric devices, optical cables, and various other domains.
References
- https://blog.thepipingmart.com/metals/metalloids-uses-and-properties/
- https://www.adda247.com/school/metalloids/
- https://study.com/learn/lesson/metalloids-on-periodic-table.html
- https://www.chemicool.com/definition/metalloid.html
- https://www.xometry.com/resources/materials/metalloid-elements/
- https://www.xometry.com/resources/materials/metalloids/
- https://www.vedantu.com/chemistry/metalloids
- https://byjus.com/chemistry/metalloids/
- https://collegedunia.com/exams/metalloids-science-articleid-6218
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/06%3A_The_Periodic_Table/6.07%3A_Metalloids
