In chemistry, compounds having two or more chiral centers or a plane of symmetry are called meso compounds. These molecules are achiral and symmetrical. They fall somewhere in between chiral and achiral, hence the name “meso” (from the Greek for “middle”) compounds.
Interesting Science Videos
What is Meso Compound?
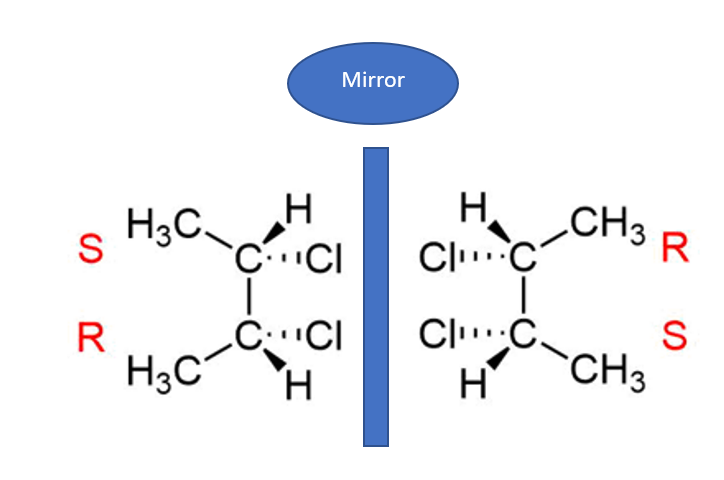
A meso compound is an achiral compound with chiral centers. A meso compound has two or more stereocenters but is optically inactive because its mirror image is a superimposition of the original.
A meso compound is a stereoisomer with two or more chiral centers but no optical activity due to an internal plane of symmetry. Mesomers are substances in which the plane-polarized light undergoes no net rotation. Thus, meso compounds are organic molecules with two mirror-image chiral carbons and zero net rotation.
Characteristics of Meso Compounds
- Achiral compounds are meso compounds.
- They have multiple chiral centers (two or more).
- They possess an internal plane of symmetry.
- This internal symmetry plane separates mesocompounds into two equal halves.
- They are mirror images that are superimposable.
- These are optically inactive because they have optical activities that are equal but opposite.
Meso Compound Examples
Meso compounds have chiral centers (sp3 hybridized tetrahedral atoms that are joined to four different groups), but most of the time they are achiral because of symmetry. As stated, a meso compound should have at least two chiral sp3 hybridized atoms, except for the nitrogen atom of a tertiary amine, and at least one internal plane that splits the molecule into two mirror copies.
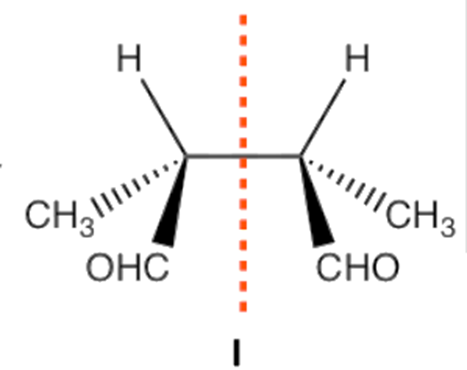
Meso compounds can manifest in several structural configurations, including but not limited to pentane, butane, heptane, and cyclobutane. It is not obligatory for there to be exclusively two stereocenters; rather, there is potential for the presence of additional stereocenters.
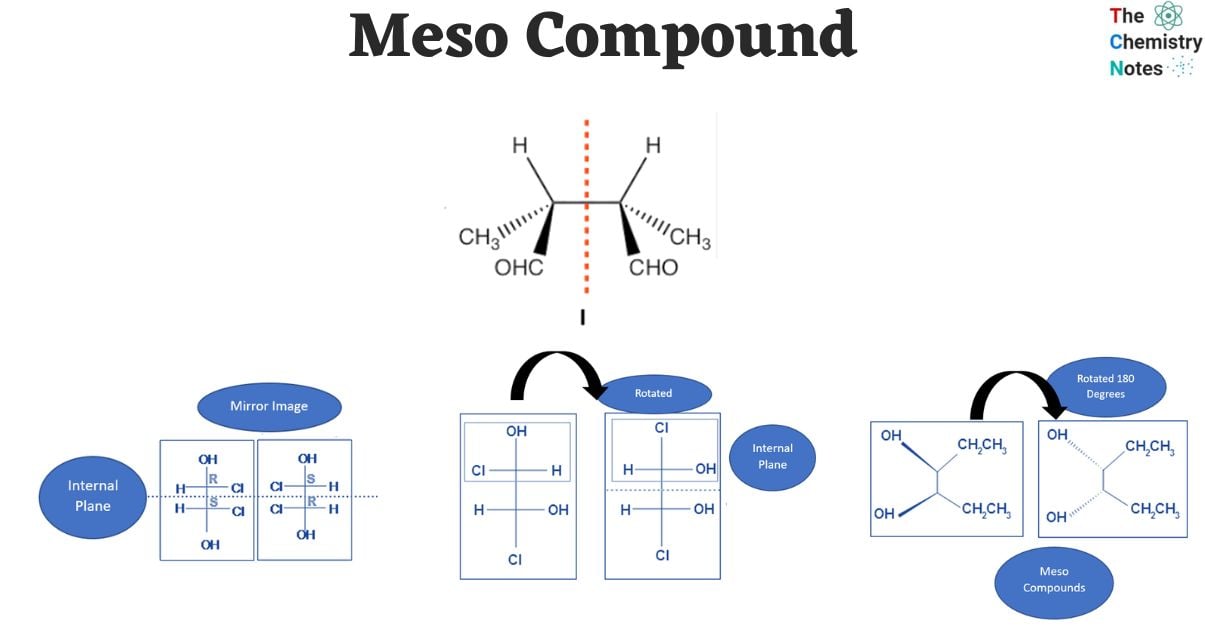
Configurations of Meso Compounds
When two chiral centers are present, there are four possible configurations for the meso compound, i.e.
- R-S configuration
- S-R configuration
- R-R configuration
- S-S configuration
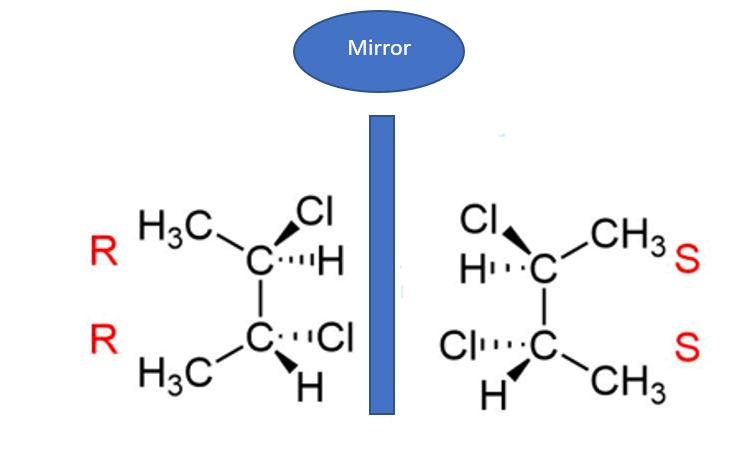
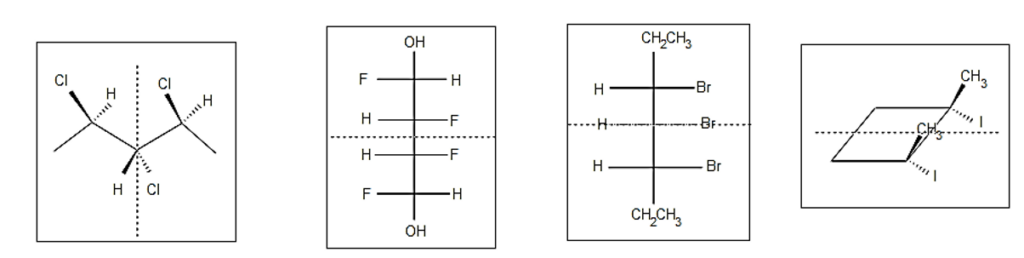
Identification of Meso Compounds
- In the event that compound A is classified as a meso compound, it is imperative that it possesses a minimum of two stereocenters, an internal plane, and exhibits the stereochemistry of both R and S configurations.
- Search for an internal plane or internal mirror situated within the compound.
- The determination of whether a compound is meso or not is heavily reliant on its stereochemistry, specifically the assignment of R or S configuration. As previously indicated, a meso compound exhibits optical inactivity due to the cancellation of its stereochemistry. In the case of a meso compound containing two stereocenters, the presence of R results in the cancellation of S.
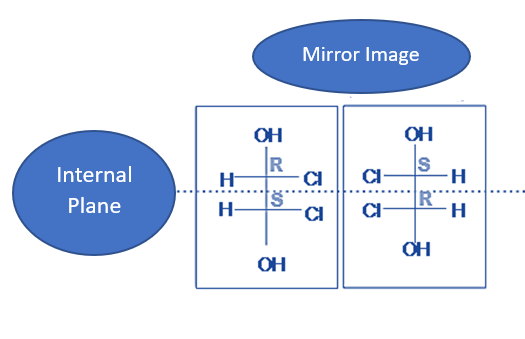
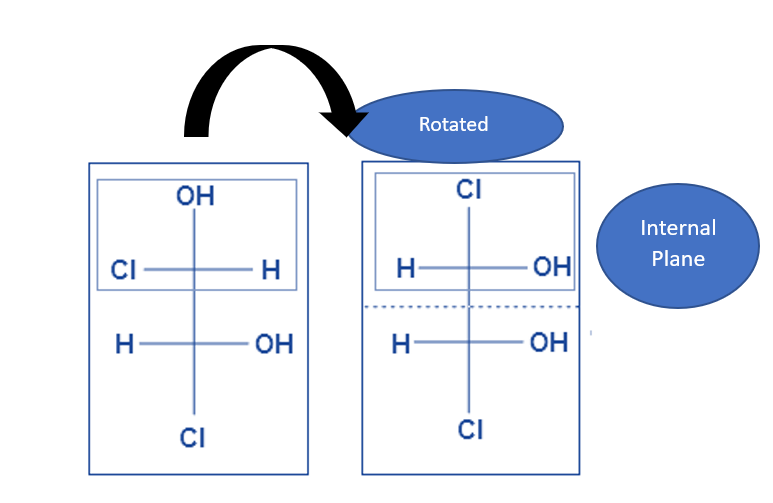
- Substituted groups linked to a stereocenter can be rotated to reveal the interior plane thanks to single bonds’ sp3-orbital character. The stereochemistry of a molecule remains the same regardless of its orientation.
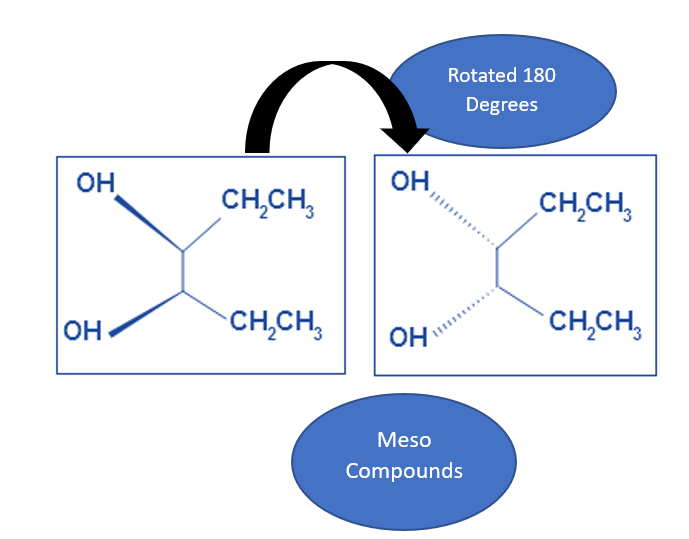
- When the entire molecule is rotated by 180 degrees, we have a second example. The two molecules down here are still meso.
Frequently Asked Questions (FAQ)
Are all achiral compounds meso?
Whether or not the molecule has a chiral center, a meso compound comprises a plane of symmetry and is therefore achiral. A plane of symmetry is a plane that divides a molecule into two halves that are mirror images of one another.
Why mesocompounds are optically inactive?
Due to the equal but opposite rotation of plane-polarized light by two isomers, they are optically inactive.
What is a chiral compound, and what does it mean?
A chiral substance rotates plane-polarized light in either the anticlockwise or clockwise direction. Chirality corresponds to optical activity, which should be a substance able to rotate polarized light in virtually any direction.
How can you determine whether a compound is optically active?
Compounds that are capable of optical rotation are referred to as optically active. All chiral compounds exhibit optical activity. The chiral compound contains a carbon atom or group that is attached to four distinct atoms or groups. It creates two mirror images that cannot be superimposed.
What are meso compounds?
Meso compounds refer to molecules that exhibit superimposition with their mirror images, even in the presence of a chiral center.
Video on Meso Compounds
References
- https://edvnce.com/meso-compound/
- https://www.chem.ucalgary.ca/courses/350/Carey5th/Ch07/ch7-2-4.html
- Vollhardt, K. P.C. & Shore, N. (2007). Organic Chemistry (5thEd.). New York: W. H. Freeman. (190-192)
- Shore, N. (2007). Study Guide and Solutions Manual for Organic Chemistry (5th Ed.). New York: W.H. Freeman. (70-80)
- https://byjus.com/chemistry/meso-compound/#
- https://www.chemistrysteps.com/symmetry-chiralty-meso-compunds/
- https://unacademy.com/content/nta-ugc/study-material/chemistry/meso-compounds/

