Mercury is a chemical element with an atomic number of 80 and is represented by the symbol ‘Hg’ in the periodic table. It is silvery-white in appearance classified as metal and belongs to the d-block of group 12 of the periodic table. Since mercury is a silver liquid, it has long been referred to as quicksilver. This is also represented by the chemical symbol. Hg stems from the Latin term hydrargyrum, which means “watery silver.”
Mercury is an exceedingly uncommon element in the Earth’s crust, with an average crustal abundance by mass of about 0.08 parts per million (ppm). Mercury has three potential electrical charge states, or valence states, in nature. Elemental mercury (Hg0) has no electric charge. Mercury may also be found in two positively charged, or cationic, states: Hg2+ (mercuric) and Hg1+ (mercurous).
Interesting Science Videos
History of Mercury
- Approximately 30,000 years ago, early humans used cinnabar (mercury sulfide) to decorate caves in France and Spain with artwork.
- The metal was frequently utilized by the ancients as a solvent to extract gold from river debris. As a liquid, mercury would dissolve gold, which could be recovered by mercury distillation.
- Mercury was known to the ancient Chinese, Egyptians, and Indians, and it has been discovered in Egyptian tombs going back to around 1500 B.C.
- Aristotle refers to mercury as ‘hydro-argyros’ in the fourth century B.C., which translates as liquid-silver or water-silver. The Romans somewhat adjusted the Greek term, referring to mercury as Hydrargyrum, from which we obtain the modern molecular symbol Hg.
- Several scientists, notably Carl Wilhelm Scheele, Joseph Priestley, and Humphry Davy, utilized mercury to find new elements.
Occurrence of Mercury
- Rarely does mercury exist in nature unbound, however, it can be found in ores, primarily mercury sulfide (cinnabar, HgS).
- The richest mercury ores contain up to 2.5% mercury by mass, while even the leanest concentrated deposits contain at least 0.1% mercury.
- It can be found as a native metal (rare) or in cinnabar, metacinnabar, sphalerite, cordierite, livingstonite, and other minerals, with cinnabar (HgS) being the most frequent ore. Mercury ores are frequently found in hot springs and other volcanic areas.
- The metal is obtained by burning cinnabar in an air current. The resultant mercury vapor is condensed in order to collect the liquid metal. The equation for this extraction is:
HgS + O2 → Hg + SO2- Mercury has 34 isotopes with known half-lives and mass numbers ranging from 175Hg to 208Hg. Natural mercury is an intricate mixture of seven isotopes.
Isotopes of Mercury
Hg, in its natural state, is observed to exist as a composite amalgamation comprising a minimum of seven distinct isotopes that include: 196Hg, 198Hg, 199Hg, 200Hg, 201Hg, 202Hg, and 204Hg.
Naturally Occurring Isotopes of Mercury
| Isotopes | Natural Abundance (% atoms) |
|---|---|
| 196Hg | 0.15 (1) |
| 198Hg | 9.97 (20) |
| 199Hg | 16.87 (22) |
| 200Hg | 23.10 (19) |
| 201Hg | 13.18 (9) |
| 202Hg | 29.86 (26) |
| 204Hg | 6.87 (15) |
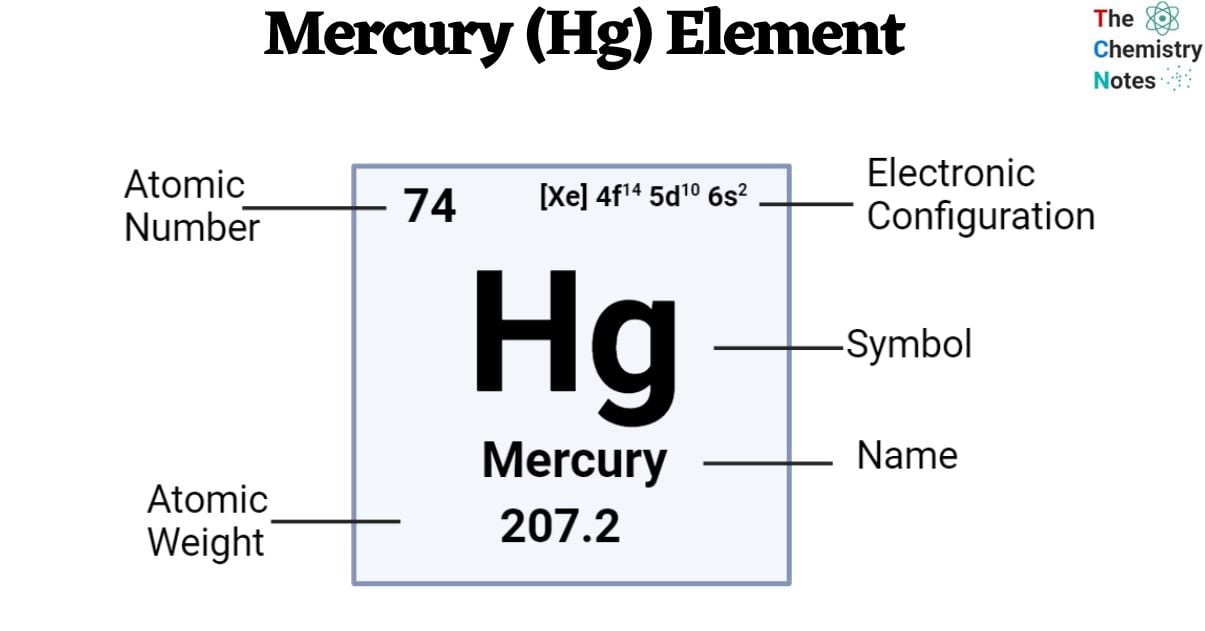
Elemental Properties of Mercury
| Electronic Configuration | [Xe] 4f14 5d10 6s2 |
| Atomic Number | 80 |
| Atomic Weight | 200.59 g.mol -1 |
| State at 20°C | Liquid |
| Group, Period, and Block | 12, 6, d-block |
| Density | 13.546 g/cm3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 32, 18, 2 |
| Electrons | 80 |
| Protons | 80 |
| Neutrons in the most abundant isotope | 120 |
Physical Properties of Mercury
- Mercury metal may be frozen and turned into a solid at -38.85 °C, and it can be converted into a gas when heated at 365.6 °C. The density of mercury is 13.59 grams per cubic centimeter.
- Mercury has two interesting physical characteristics. For starters, it has a very high surface tension. Surface tension is a feature of liquids that causes them to behave as though they are covered with skin.
- Solid mercury is ductile and malleable in nature. It can be sliced with a knife.
- Mercury is also an excellent conductor of electricity. This feature is utilized in a variety of practical devices.
- When compared to other metals, it is a poor heat conductor.
| Color/physical appearance | shiny, silvery-white |
| Melting point/freezing point | 234.3210 K (−38.8290 °C, −37.8922 °F) |
| Boiling point | 629.88 K (356.73 °C, 674.11 °F) |
| Density | 13.546 g/cm3 |
| Malleability | Yes (when solid) |
| Ductility | Yes (when solid) |
| Electronegativity | 2.0 (Pauling Scale) |
Chemical Properties of Mercury
- In dry air, the metal is rather stable, but in humid air, it progressively tarnishes to produce a gray oxide layer.
- Mercury does not react with weak acids; instead, it is oxidized by strong acids.
- Compounds of mercury can be found in two oxidation states: +1 and +2.
- Mercury and other metals, such as tin, silver, gold, and amalgams, mix easily to form alloys. Mercury is typically stored in iron containers since it does not amalgamate with iron.
- It interacts with several hot acids, but not with most cold acids.
Chemical Reactions of Mercury
- The Reaction of Mercury With Air
At room temperature, mercury does not react with oxygen. When heated, it slowly oxidizes, generating mercury(II) oxide.
when heated Δ
2 Hg (s) + O2 (g) → 2 HgO (s) [red]
- The Reaction of Mercury With Water
Under normal conditions, mercury does not react with water.
- The Reaction of Mercury With Halogens
Fluorine, F2, and mercury metal combine to generate the dihalide mercury(II) fluoride, abbreviated HgF2.
Hg (l) + F2 (g) → HgF2 (s) [white]Chlorine, Cl2, and mercury metal combine to generate the dihalide mercury(II) chloride, HgCl2.
Hg (l) + Cl2 (g) → HgCl2 (s) [white]The dihalide mercury(II) bromide, HgBr2, is created when mercury metal interacts with bromine, Br2.
Hg (l) + Br2 (l) → HgBr2 (s) [white]The dihalide mercury(II) iodide, HgI2, is created when mercury metal and iodine, I2, react.
Hg (l) + I2 (s) → HgI2 (s) [red]Mercury doesn’t react with non-oxidizing acids, but it reacts with concentrated nitric acid (HNO3) or concentrated sulfuric acid (H2SO4) to create compounds that include mercury(II) and either nitrogen or sulfur oxides.
3 Hg (l) + 8 H+ (aq) + 2 NO3− (aq) → 3 Hg2+ (aq) + NO (g) + 4 H2O (l)The slow breakdown of mercury in weak nitric acid yields mercury(I) nitrate, often known as mercurous nitrate or Hg2(NO3)2.
Hg (l) + 2 H2SO4 (aq) → Hg2+ (aq) + SO42− (aq) + SO2 (g) + 2 H2O (l)Uses of Mercury
Used in Laboratory Devices
Mercury has advantages due to its high density and linear thermal expansion, which are used in sphygmomanometers, barometers, diffusion pumps, and other laboratory equipment. Mercury is used in manometers and barometers, which measure the pressure of gases and liquids, due to its high density.
Used For Chlorine Production
The most major application of mercury is in the production of chlorine. An electric current is sent through sodium chloride to make chlorine. However, there is a disadvantage to employing this strategy. The metal sodium (Na) is very reactive. If there is any water present, the sodium will react aggressively with it. This reaction makes chlorine generation considerably more difficult.
Used in Electronic Devices
Mercury is commonly utilized in the manufacture of advertising signs, mercury switches, and other electrical devices. It is also utilized in mercury-vapor lamps (which create light with a high concentration of UV rays). These lamps are commonly used for street lighting, sun lamps, and UV (black light) lamps.
Miscellaneous Uses
Mascara includes thiomersal, a form of mercury. Mercury(II) fulminate is employed as a cartridge primer in guns. Mercury was used as a propellant in ion engines due to its high liquid density, low ionization energy, and other features.
Health Effects of Mercury
- Inhalation of mercury vapors and buildup near the neurological system disturbs its functioning and can potentially destroy brain function.
- Organic mercury compounds are exceedingly poisonous in nature; they should all be handled with caution and only by skilled individuals. Even a few drips can kill the individual.
- Contact with mercury produces allergic responses, skin rashes, headaches, and fatigue.
- Mercury buildup in reproductive organs causes sperm destruction and miscarriages. If a baby is born, birth problems are possible.
Environmental Effects of Mercury
- Mushrooms may get mercury buildup from the soil.
- The level of mercury in acidic surface waters might be rather high.
- Once mercury has entered surface waters or soils, bacteria can change it into methyl mercury, a chemical that can be easily absorbed by most creatures and is known to cause nerve damage
- Fish are living things that daily take in large levels of methyl mercury from surface waters. Methyl mercury can consequently build up in fish and the food systems to which they belong.
Video References
References
- https://byjus.com/chemistry/mercury/
- https://energyeducation.ca/encyclopedia/Mercury_(element)
- https://www.lenntech.com/periodic/elements/hg.htm
- https://www.chemicool.com/elements/mercury.html
- https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/chemical-properties.html
- https://www.chemistrylearner.com/mercury.html
- https://chemicalengineeringworld.com/mercury-element-properties-and-information/
- https://pilgaardelements.com/Mercury/Reactions.htm
- https://www.webelements.com/mercury/chemistry.html

