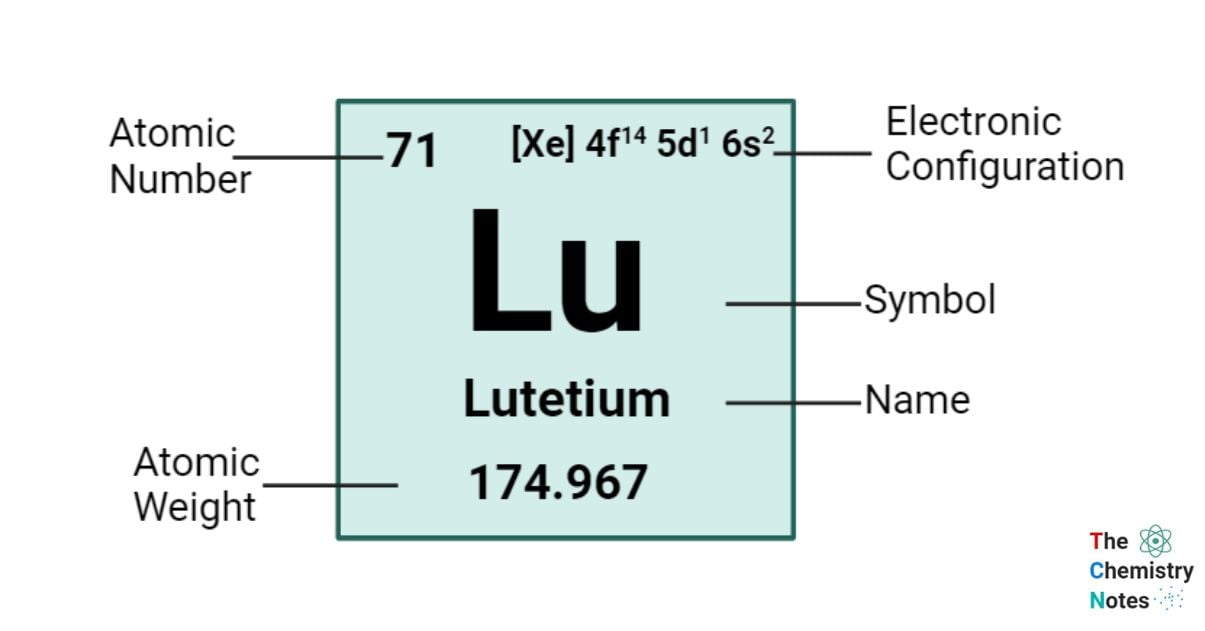
Lutetium is a chemical element with an atomic number of 71 and is represented by the symbol ‘Lu’ in the periodic table. It is hard and silvery in appearance and classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. Lutetium is commonly encountered in its trivalent oxidation state, akin to the majority of other elements belonging to the lanthanide series. However, it is also possible for it to exist in the 0, +1, and +2 states.
Lutetium is not naturally occurring in its free elemental form but rather exists within various minerals, predominantly monazite.
Interesting Science Videos
History of Lutetium
- The mineral gadolinite, also known as yttrium, was first discovered in 1794. Since its discovery, it has served as a primary source for rare earth elements such as yttria, terbia, erbia, and others.
- The erbia compound was effectively divided into two separate constituents, specifically ytterbia, and erbia, by the accomplished Swiss chemist Jean-Charles Marignac. Ytterbia was commonly perceived as a compound comprising ytterbium.
- In the year 1907, Georges Urbain, during his experimental endeavors conducted at the esteemed Sorbonne University in Paris, achieved the successful isolation of ytterbium into two distinct elements. These elements were subsequently bestowed with the names lutecium and neo-ytterbium by Urbain himself.
- The elements, originally given different names, were subsequently renamed lutetium and ytterbium by chemists.
- The element was separated from ytterbia by Carl Auer von Welsbach, who then named it Cassiopeium after the circumpolar constellation Cassiopeia.
- Charles James conducted a successful extraction of an element at the University of New Hampshire.
- The element’s discovery is attributed to Georges Urbain, Charles James, and Carl Auer von Welsbach. However, Urbain is credited as the discoverer due to being the first to report the results, despite the independent discoveries made by the other two scientists.
- The nomenclature of the element lutetium is derived from its etymological origin in Lutetia, the Latin appellation for the city of Paris.
Occurrence of Lutetium
- Lutetium is not naturally occurring in its elemental form but rather exists within various minerals, predominantly monazite.
- Throughout history, the process of isolating rare earth elements from one another has posed significant challenges due to their closely resembling chemical properties. This has resulted in substantial costs associated with their isolation.
- Since the 1940s, the cost of production has been reduced due to the development of ion exchange and solvent extraction techniques.
- The process of obtaining pure lutetium metal entails the reduction of the anhydrous fluoride compound through the utilization of calcium metal.
- Lutetium exhibits a total of 35 isotopes, spanning mass numbers 150 to 184, for which the respective half-lives have been determined. The presence of two isotopes is observed in naturally-occurring lutetium.
Isotope of Lutetium
Lutetium is found naturally on Earth in the form of two isotopes, namely lutetium-175 and lutetium-176. Among these two options, only the former exhibits stability, thereby classifying the element as monoisotopic.
Naturally Occurring Isotopes of Lutetium
| Isotopes | Natural Abundance (%atoms) |
|---|---|
| 175Lu | 97.4 |
| 176Lu | 2.6 |
Elemental Properties of Lutetium
| Electronic Configuration | [Xe] 4f14 5d1 6s2 |
| Atomic Number | 71 |
| Atomic Weight | 174.97 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Lanthanides, 6, f-block |
| Density | 9.8 g/cm3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 32, 9, 2 |
| Electrons | 71 |
| Protons | 71 |
| Neutrons in the most abundant isotope | 104 |
Physical Properties of Lutetium
- Terbium has an atomic number of 65 and is a silvery-white rare earth metal. It has a melting point of 1652 °C (3006 °F) and a boiling point of 3402 °C (6156 °F).
- Tb has a solid phase density of 9.841 g/cm3 and a liquid or molten phase density of 9.3 g/cm3.
- It is malleable which means it can be easily beaten into thin sheets without any cleavage.
- It is ductile which means it is possible to draw thin wires from it without breaking.
- The hardness of metals within the lanthanide series exhibits a positive correlation with atomic number. Consequently, Lutetium, possessing a higher atomic number, is classified as a highly durable metal.
- In chemical compounds, lutetium typically manifests in the trivalent state, denoted as Lu3+. The majority of its salts exhibit a lack of coloration.
- The phenomenon of paramagnetism can be observed in ions of the lanthanide elements. Nevertheless, Lu+3 does not exhibit paramagnetism due to the presence of 14 electrons in its f orbital, specifically f14.
- Lutetium exhibits a notably diminished value of third ionization enthalpy. The phenomenon can be attributed to the inherent stability of vacant or partially occupied orbitals.

| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1925 K (1652 °C, 3006 °F) |
| Boiling point | 3675 K (3402 °C, 6156 °F) |
| Density | 9.841 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.27 (Pauling Scale) |
Chemical Properties of Lutetium
- Lutetium exhibits reactivity by undergoing reactions to produce various compounds such as acetates, sulfates, fluorides, oxides, hydroxides, oxalates, carbonates, phosphates, and so forth.
- The formation of lutetium hydroxide occurs as a result of the reaction between lutetium and water. The rate of reaction is reduced at lower temperatures and increased at higher temperatures in the presence of water.
- Lutetium metal exhibits reactivity with various halogens, resulting in the formation of trihalides. Out of the given substances, it is noteworthy that only fluoride exhibits the property of solubility in water.
- Carbides are produced through the process of heating metals in the presence of carbon.
- Lutetium compounds are known to display a +3 oxidation state.
- The lanthanides exhibit reactivity with hydrogen upon moderate heating.
- Lutetium exhibits a pronounced propensity to undergo combustion, resulting in the formation of lutetium oxide, a chemical compound, when subjected to a temperature of 150°C.
- Lutetium demonstrates a remarkable capacity to resist corrosion in the presence of dry air, while concurrently displaying a proclivity to undergo tarnishing when exposed to moist air.
Chemical Reaction of Lutetium
- The Reaction of Lutetium With Air
Lutetium exhibits a gradual oxidation process when exposed to atmospheric conditions, resulting in the formation of lutetium (III) oxide, denoted as Lu2O3, which readily combusts.
4 Lu (s) + 3 O2 (g) → 2 Lu2O3 (s)
- The Reaction of Lutetium With Water
Lutetium exhibits a sluggish reactivity when exposed to cold water, while its reaction rate is significantly accelerated when subjected to hot water. This chemical process results in the formation of lutetium(III) hydroxide, denoted as Lu(OH)3, along with the liberation of hydrogen gas (H2).
2 Lu (s) + 6 H2O (g) → 2 Lu(OH)3 (aq) + 3 H2 (g)
- The Reaction of Lutetium With Halogens
The element lutetium exhibits a propensity to engage in chemical reactions with various halogens, resulting in the formation of lutetium (III) halides.
The chemical reaction between lutetium metal and fluorine gas (F2) results in the formation of lutetium (III) fluoride, denoted as LuF3.
2 Lu (s) + 3 F2 (g) → 2 LuF3 (s) [White]
The chemical reaction between lutetium metal and chlorine gas (Cl2) results in the formation of lutetium (III) chloride, denoted as LuCl3.
2 Lu (s) + 3 Cl2 (g) → 2 LuCl3 (s) [White]
The chemical reaction between lutetium metal and bromine (Br2) results in the formation of lutetium (III) bromide, denoted as LuBr3.
2 Lu (s) + 3 Br2 (g) → 2 LuBr3 (s) [White]
The chemical reaction between lutetium metal and iodine represented as I2, results in the formation of lutetium (III) iodide, denoted as LuI3.
2 Lu (s) + 3 I2 (g) → 2 LuI3 (s) [Brown]
- The Reaction of Lutetium With Acid
Lutetium exhibits high solubility in dilute sulphuric acid, resulting in the formation of colorless Lu(III) ions and the liberation of hydrogen gas, denoted as H2.
2 Lu (s) + 3 H2SO4 (aq) → 2 Lu3+ (aq) + 3 SO42- (aq) + 3 H2 (g)
Uses of Lutetium
Numerous significant applications exist for lutetium and its compounds some of which are discussed here:
Used As Catalyst
Lutetium is occasionally employed as a catalyst within the petroleum industry. A catalyst is a substance employed to enhance or inhibit the rate of a chemical reaction. The catalyst remains unchanged throughout the course of the reaction. Lutetium finds applications in various industrial processes such as petroleum cracking, polymerization, hydrogenation, and alkylation.
Used As Dopant
Magnetic bubble memory devices utilize gadolinium gallium garnet doped with lutetium as an indispensable component for their operational functionality. Lutetium aluminum garnet (LuAG) exhibits promising characteristics that render it a viable candidate for utilization as a lens material within the domain of high refractive-index immersion lithography. Moreover, it exhibits practicality as a phosphor compound employed in the fabrication of LED light bulbs.
Used In Detection
Lutetium oxy orthosilicate (Lu2SiO5:Ce) serves as a scintillation crystal material in positron emission tomography (PET) detectors, enabling the acquisition of medical scans to generate three-dimensional depictions of cellular activity within the human body. The preferred compound utilized as detectors in positive emission tomography (PET) is cerium-doped lutetium oxyorthosilicate.
Used For Its Radioactivity
Lutetium-176 serves as a highly valuable pure-beta emitter, exhibiting exceptional utility in the field of meteorite dating. The synthetic isotope known as lutetium-177 is utilized in the field of radionuclide therapy. The utilization of lutetium-177 as a radionuclide in the treatment of neuroendocrine tumors and the alleviation of bone pain is experiencing a notable surge. According to scholarly research, it has been established that lutetium-ion atomic clocks possess the potential to surpass the precision levels exhibited by currently available atomic clocks.
Health Effects of Lutetium
- Lutetium exhibits a limited degree of toxicity and lacks established biological roles within the human organism. However, there is speculation regarding its potential capacity to enhance metabolic processes. Furthermore, it is widely postulated that there exists a potential risk of explosion.
- The consumption of insoluble lutetium salts does not present a toxic hazard, while lutetium in its pure form and soluble lutetium salts demonstrate a mild level of toxicity. Several compounds containing lutetium have been found to possess notable toxicity, thus requiring careful handling protocols.
- Lutetium particulates possess the inherent capacity to pose a potential risk of fire. Accidental ignition of these substances within the workplace can result in the infliction of severe burns.
Environmental Effects of Lutetium
- Lutetium does not present any discernible environmental hazards to the flora and fauna.
Video on Lutetium
References
- https://byjus.com/chemistry/lutetium/#:~:text=Lutetium%20is%20a%20chemical%20element,on%20the%20list%20of%20lanthanides.
- https://www.rsc.org/periodic-table/element/71/lutetium
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements, Oxford University Press, New York, 2nd Edition, 2011.
- Thomas Jefferson National Accelerator Facility – Office of Science Education, It’s Elemental – The Periodic Table of Elements, accessed December 2014.
- https://www.chemicool.com/elements/lutetium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Lutetium
- https://www.lenntech.com/periodic/elements/lu.htm

