Lithium aluminum hydride (LAH) is a reagent used widely in organic synthesis for reduction. LAH reacts strongly with H2O in an exothermic reaction that results in the potentially harmful release of H2 gas. LAH also quickly interacts with protic solvents. Despite its limited solubility, LAH is commonly utilized in ether and THF.
It is a white solid discovered in 1947.This chemical is utilized as a reducing agent in organic synthesis, particularly for the reduction of esters, carboxylic acids, and amides. Aldehydes can be converted to primary alcohols, ketones to secondary alcohols, carboxylic acids and esters to primary alcohols, amides and nitriles to amines, epoxides to alcohols, and lactones to diols using the LiAlH4 reagent.
Interesting Science Videos
Properties of Lithium Aluminum Hydride (LiAlH4)
| Chemical Formula | LiAlH4 |
| Molar Mass | 37.95 g·mol−1 |
| Appearance | White Crystals (pure samples) Grey Powder (commercial material) |
| Odor | Odorless |
| Melting Point | 150 °C (302°F) 423K (decomposes) |
| Boiling Point | 184℃ |
| Density | 0.917 g/cm3 (solid) |
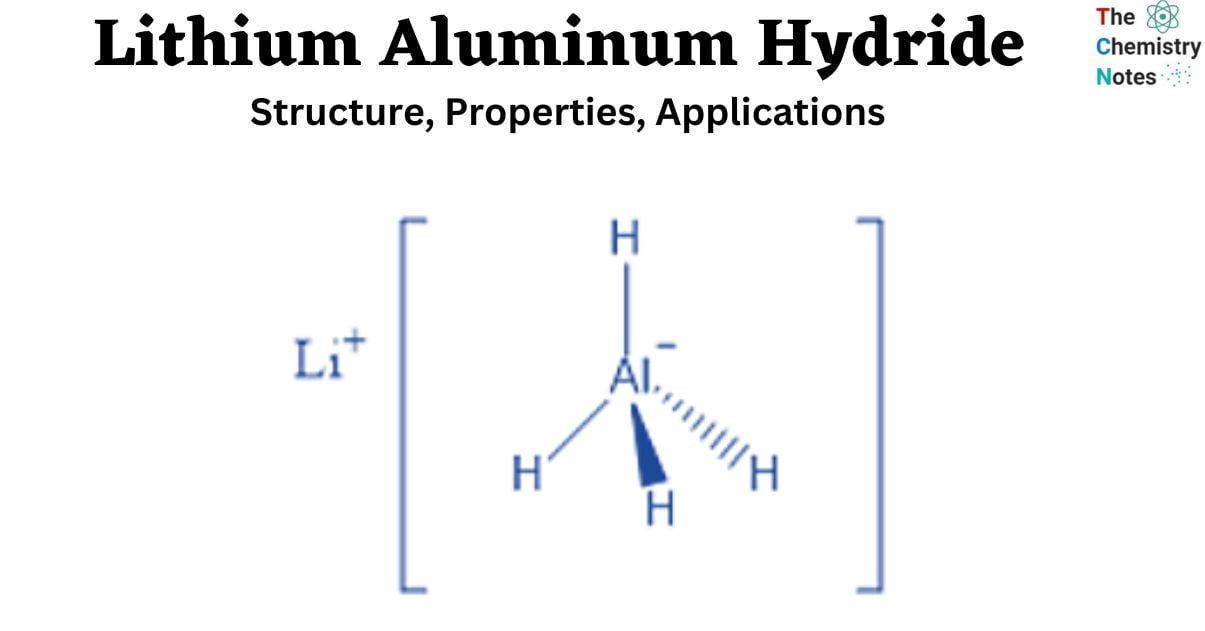
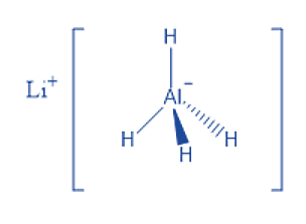
Structure of Lithium Aluminum Hydride (LiAlH4)
The hydrogens in aluminum hydride, the AlH4– ion, are grouped in a tetrahedral pattern around Al3+. It is formed via the coordination of hydride H– ions with Al3+ ions. Five AlH4– tetrahedra surround the Li+ centers in the structure. The Li+ centers are connected to one hydrogen atom from each of the surrounding tetrahedra to produce a bipyramid configuration.
Synthesis of Lithium Aluminum Hydride (LiAlH4)
- The reaction between lithium hydride and aluminum chloride results in lithium aluminum hydride.
4 LiH + AlCl3 → LiAlH4 + 3 LiCl- Furthermore, industrial synthesis necessitates the first preparation of sodium aluminum hydride at high pressure and temperature.
Na + Al + H2 → NaAlH4- Following that, LiAlH4 is produced using a salt metathesis process as follows:
NaAlH4 + LiCl → LiAlH4 + NaCl- It progresses with a high yield. To remove LiCl from an ethereal solution of LAH, filtration is utilized.
Chemical Properties of Lithium Aluminum Hydride (LiAlH4)
- When it comes into contact with water, it produces hydrogen gas. As a result, it should not be exposed to moisture, and the reactions should take place in an inert and dry atmosphere. In the laboratory, this procedure might be used to produce hydrogen.
LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2- The reduction reaction must be carried out in anhydrous non protic solvents such as diethyl ether, THF, and so on.
- It dissolves easily in diethyl ether. However, because of the existence of catalytic impurities, it may spontaneously breakdown. THF, despite its poor solubility, is the recommended solvent for LAH.
- Normally, the reactions are carried out with an excess of LiAlH4. To eliminate any remaining moisture, a tiny quantity of the reagent is added to the solvent.
- The reaction mixture is cooled in an ice bath first, and then the lithium aluminum hydride is quenched by a cautious and very gradual addition of ethyl acetate, followed by methanol, and lastly cold water.
- Sometimes the reagent is degraded by progressively adding undried solvent and then diluting sulfuric acid to the reaction mixture.
Why Lithium Aluminum Hydride (LiAlH4) is Effective?
- Aluminum is an electronegativity-limited metal. As a result, the Al-H bond is highly polarized, with Al+ positively charged and H– negatively charged.
- The anomalous polarization (and oxidation state of -1) of typically positive hydrogen leads in increased reactivity.
- The anomalous polarization (and oxidation state of -1) of typically positive hydrogen leads in significant reactivity, particularly with atoms that may receive electrons (be reduced), allowing the hydrogen to revert to its regular oxidation state of +1.
- It also combines with other positive centers, especially slightly acidic hydrogens found in alcohols, water, carboxylic acids, or alkynes, to form hydrogen gas, which is very combustible and quickly bursts.
Application of Lithium Aluminum Hydride (LiAlH4)
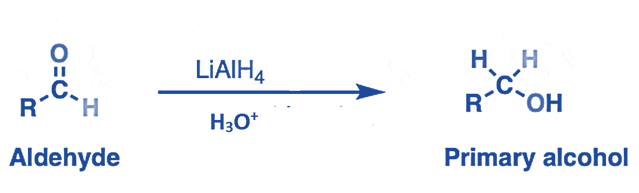
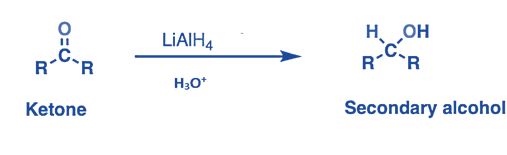
- Aldehydes or ketones are converted to the equivalent primary or secondary alcohols by LiAlH4.
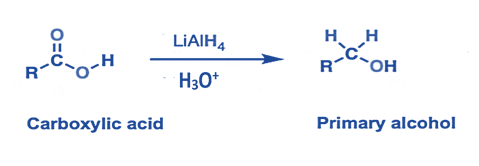
- Lithium aluminum hydride is used to convert carboxylic acids, esters, and acid halides to primary alcohols.
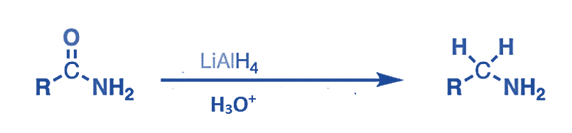
- The amides are converted to amines by lithium aluminum hydride, LiAlH4. This technique is very beneficial for producing secondary amines.
- The nitriles are reduced to primary amines by LiAlH4.
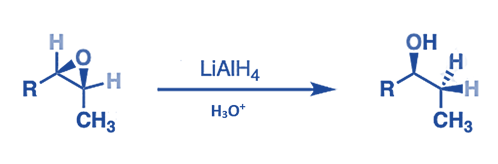
- Lithium aluminum hydride converts oxiranes (epoxides) to alcohol. The procedure includes a hydride attack on the less inhibited side of the epoxide.
- Haloalkanes and haloarenes are converted to hydrocarbons using lithium aluminum hydride.
Frequently Asked Questions (FAQ)
What is LiAlH4 used for?
LiAlH4 is a powerful reducing agent that is commonly employed in organic chemistry.
How is LiAlH4 stored?
It is stored in firmly sealed containers in a cold, dry environment away from flammable items.
Why is LiAlH4 such a powerful reducing agent?
Due to the less electronegative nature of aluminum and the more polar nature of the Al-H bond in LiAlH4, this compound is a strong reducing agent
When LiAlH4 is introduced to water, what happens to its pH?
The H–, hydride ions can aggressively react with water to liberate hydrogen gas, causing the solution to become alkaline and include LiOH and Al(OH)3. As a result, the pH rises more than 7. The following illustration depicts the reaction of lithium aluminum hydride, LiAlH4, with water.
LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2
Since it has a strong basicity, it can react with protic solvents like methanol in addition to water.
Video on Lithium Aluminum Hydride
References
- https://byjus.com/chemistry/lithium-aluminium-hydride/
- https://www.masterorganicchemistry.com/2023/02/03/lialh4-lithium-aluminum-hydride/
- https://www.organic-chemistry.org/chemicals/reductions/lithiumaluminumhydride-lah.shtm
- https://commonorganicchemistry.com/Common_Reagents/Lithium_Aluminum_Hydride/Lithium_Aluminium_Hydride.htm

