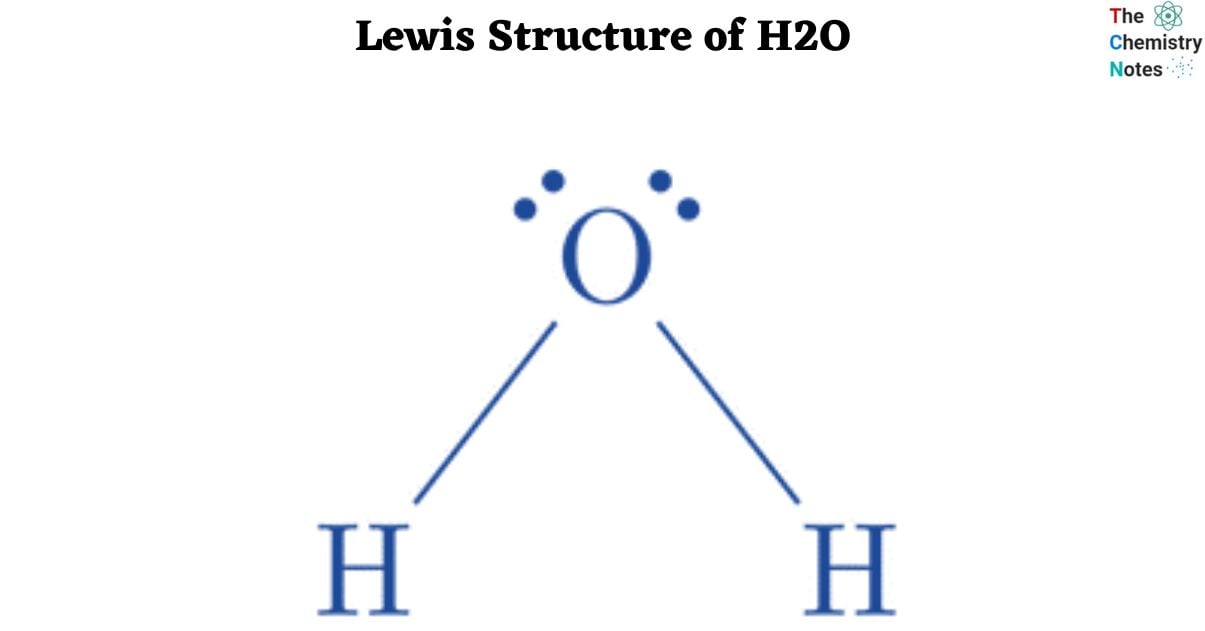
The Lewis structure represents the valence electrons of an atom or molecule. The Lewis Structure of H2O molecule reveals that two hydrogen atoms are linked to one oxygen atom. A single covalent link is formed when each hydrogen atom and oxygen atom share one electron. Furthermore, the oxygen atom has two lone pairs of electrons that are not shared by any other element. The Lewis structure of H2O shows the atom bonding arrangement and electron distribution.
Molecular Geometry of Water
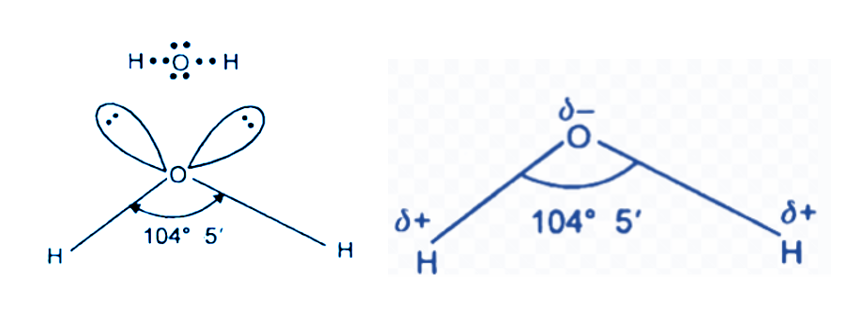
Lone pair of electrons: Two unpaired electron sets are positioned at the vertices of the molecule, and these electron pairs remain unbound to adjacent hydrogen atoms. These lone pairs originate from the valence shell of the oxygen atom.
Geometry: The molecular geometry takes on a V-shape due to the occupation of two vertices in the water molecule. This configuration results in a non-linear structure for the molecule.
Angle: According to the VSEPR theory, scientists can anticipate the angle between the hydrogen atom bonds. While a typical tetrahedral molecule exhibits a 109-degree angle, the water molecule deviates with a smaller angle, specifically between 104.5 and 109 degrees. In comparison, an ammonium molecule displays a larger angle.
Bent structure: The individual water molecule adopts a bent geometric structure. The presence of a pair of lone electrons is necessary for a molecule to exhibit a bent shape, as these lone electron pairs repel the hydrogen bonds, shaping the overall molecule into a V-shaped form.
Lewis Structure: The water molecule’s structural arrangement, known as the Lewis Structure, dictates the utilization of a total of 8 valence electrons in forming bonds within the triatomic water molecule, consisting of two hydrogen atoms and one oxygen atom.
Tetrahedral bond: The entire molecule has a tetrahedral configuration, considering the presence of 8 electrons in the outermost shell. This arrangement leads to the formation of two hydrogen bonds and two pairs of lone electrons in the outer shell.
How to Draw Lewis Structure of H2O
The following instructions should be followed while drawing the Lewis structure for H2O.
- Determine the total number of electrons in the oxygen and hydrogen atoms’ valence shells.
Within the periodic table:
- Group IA element hydrogen has a single electron in its outermost (valence) shell.
- Group VIA element oxygen has six electrons in the outermost shell.
In light of this, 1(2) + 6 = 8 valence electrons are needed overall to depict the Lewis structure of H2O.
- Calculate the total number of electron pairs in bonds and lone pairs.
Total electron pairs are calculated by dividing the total valence electron count by two. There are four electron pairs overall in the valence shells of H2O atoms.
- Identifying the central atom.
Being the center atom necessitates the ability to have a higher valence. Thus, oxygen will serve as the main atom.
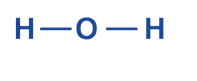
- Find the atoms that have lone pairs
Four electron pairs in all, two of which are already H-O bonds found in the schematic structure.
Two more electron pairs need to be labeled on atoms. Since the outer shell of hydrogen atoms can only contain two electrons, these cannot be on the atoms.
As a result, the two lone pairs electron are indicated on the center element, oxygen.
\
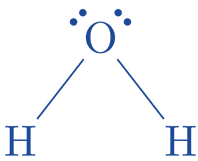
- Label any atoms that have charges on them: Atoms of hydrogen and oxygen are not charged.
- To obtain the optimal Lewis structure, minimize the charges on the atoms by forming bonds out of lone pairs: There’s no need to remove charges because the atoms are charge less. The ideal Lewis structure for H2O already exists.
- Examine each atom’s stability.
You can do this by using the following formula:
Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
The above-mentioned Lewis structure of H2O is the most appropriate and stable because there is no general formal charge.
Video on Lewis Structure of H2O
References
- https://terpconnect.umd.edu/~wbreslyn/chemistry/Lewis-Structures/lewis-structure-for-H2O.html
- https://kemicalinfo.com/articles/h2o-lewis-structure-geometry/
- https://testbook.com/chemistry/lewis-structure-h2o
- https://byjus.com/chemistry/lewis-structure-h2o/

