Lewis structures are important because they make it easy to see how atoms are arranged in molecules and provide insight into how molecules interact. They are helpful in the prediction of a molecule’s polarity, structure, and reactivity as well as its potential behavior in specific chemical interactions. Understanding the Lewis structure of CO2 so helps us understand how CO2 behaves in various conditions.
Lewis Structure of CO2
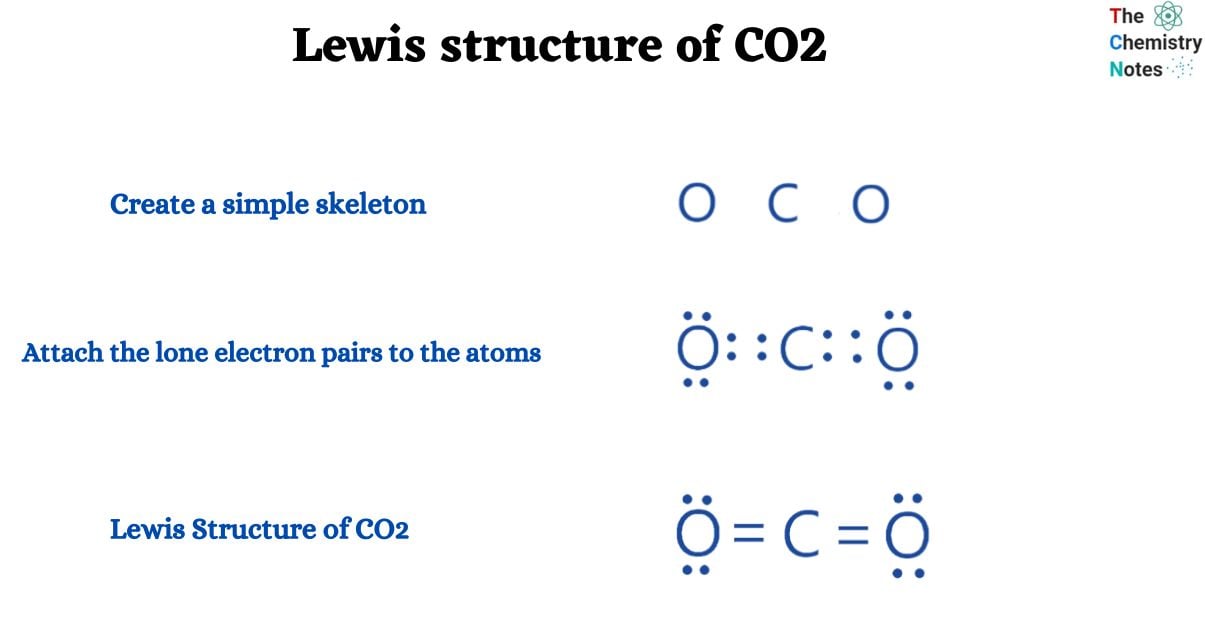
CO2 has a total of 16 valence electrons (four for carbon and two for oxygen), which are organized as O=C=O. In their outermost shells, both oxygen and carbon atoms require 8 electrons to complete an octet.
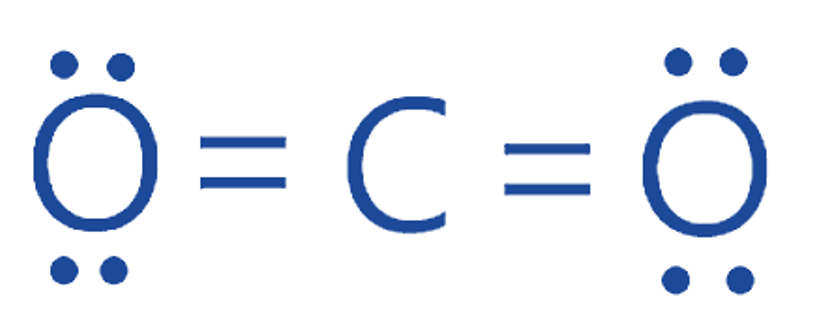
Both oxygen atoms share two electrons with the carbon atom to form two double bonds (O=C), which can also be represented by simply inserting four dots for a double bond, as seen in the image above.
How to Draw Lewis Structure of CO2
- Determine the total number of valence electrons.
CO2 is made up of two atoms: carbon (C) and oxygen (0). So, first, we must determine the valence electrons of these two atoms independently.
Valence electrons of carbon atom = 4
Valence electron of oxygen atom = 6
We have one carbon atom and two oxygen atoms in this molecule. Now,
Total number of valence electrons: 4 + 6*2 = 16.
- Total the number of valence electron pairs.
The total number of valence electrons is equal to the sum of the sigma bonds, pi bonds, and lone pair present at the valence shells, as we know. However, it is simple to compute by dividing the total number of valence electrons by two. Since total valence electrons are 16, total number of valence electrons pairs are 16/2 is 8.
- Find the central atom
Finding the central atom is one of the difficult parts of drawing a Lewis structure, however as stated in the how to draw a Lewis structure tutorial, there is a simple approach for selecting the central atom that definitely saves extra time. Because the carbon atom is the smallest in number, it will be the central atom, surrounded by two oxygen atoms on either side.
- Create a simple skeleton
We can simply design a simple structure with a carbon atom in the center surrounded by two oxygen atoms on either side.

- Attach the lone electron pairs to the atoms

- Let’s attach lone pairs of electrons to atoms now. To do this, you must ensure that every atom except the central atom has 8 electrons in order to fulfill the octet rule (hydrogen is an exception because it follows duplet rules).
- Place 6 electrons (three lone pairs of electrons) on each carbon atom and 4 electrons (two lone pairs of electrons) on the carbon atom.
- All sixteen valence electrons were used. Check whether all atoms on their outer shell are completing an octet or not; they are not. Let’s see whether we can complete the octet by sharing a single pair of electrons. So, let’s share two electron pairs between Carbon and Oxygen. Each atom now has 8 electrons on its outermost shell, completing their octet.
- Identifying formal charge on the atom
Each atom’s formal charge can be determined as follows:
Formal charge = Valence electrons (V) – Lone electron pair (L) – Bond electron pair (B)/2.
As a result, the molecule has no charge. Finally, this is a diagram of the Lewis structure of CO2.
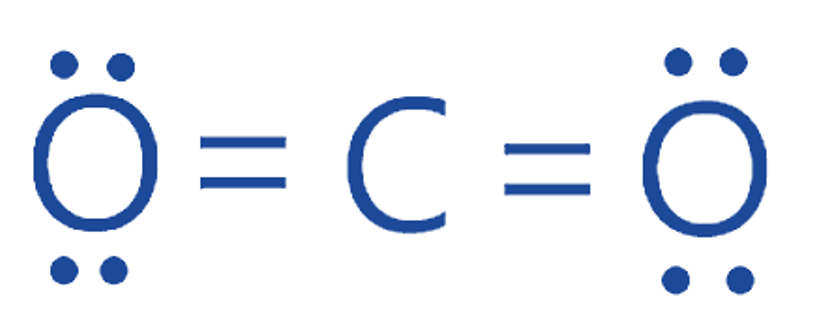
Molecular Geometry of CO2
The carbon atom in the center of a CO2 molecule is doubly bonded with two oxygen atoms on either side. The center carbon atom has no lone pairs of nonbonding electrons while both oxygen atoms have two lone pairs of nonbonding electrons. Because of the symmetrical structure, the presence of the identical atoms on either side of the central carbon atom cancels out the charge distribution. As a result, CO2 has a linear molecule geometry.
VSEPR
There are a total of 16 valence electrons, according to VSEPR theory, of which four are contributed by C and twelve by two O.
According to the Lewis structure of carbon, there are no lone pairs of electrons present.
In addition, four pairs of electrons participate in the two C=O formations, indicating that there are a total of four pairs of electrons pairs present, resulting in two sigma bonds.
However, because of the symmetrical structure, the effects of lone pairs of oxygen atoms are negated, and the CO2 molecule adopts linear molecular geometry and has a linear form.
According to the VSEPR model, a basic triatomic molecule like CO2 has a structure AX2 and no lone pair of electrons, therefore two chemical bonds extend in opposite directions, resulting in a linear molecule.
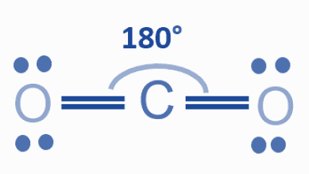
Bond Angle
Due to the linear molecular structure of CO2 and its O=C=0 arrangement, the CO2‘s bong angle is 180°. The molecules of planer shaped geometry, also known as linear geometry, always have a 180° bond angle.
Video on Lewis Structure of CO2
References
- https://byjus.com/chemistry/lewis-structure-co2/#
- https://learnwithdrscott.com/co2-lewis-structure/
- https://testbook.com/chemistry/lewis-structure-of-co2
- https://geometryofmolecules.com/co2-lewis-structure-molecular-geometry-hybridization/
- https://unacademy.com/content/jee/study-material/chemistry/co2-molecular-geometry-and-bond-angles/
- https://www.sciencecoverage.com/2020/12/co2-lewis-structure.html
- https://pediabay.com/co2-lewis-structure/

