The Lewis structure of a molecule depicts the arrangement of atoms and valence electrons. The Lewis structure of CH4 is a basic but important as it helps us understand the form and chemical activity of the molecule by highlighting the electron sharing between the carbon (C) and hydrogen (H) atoms.
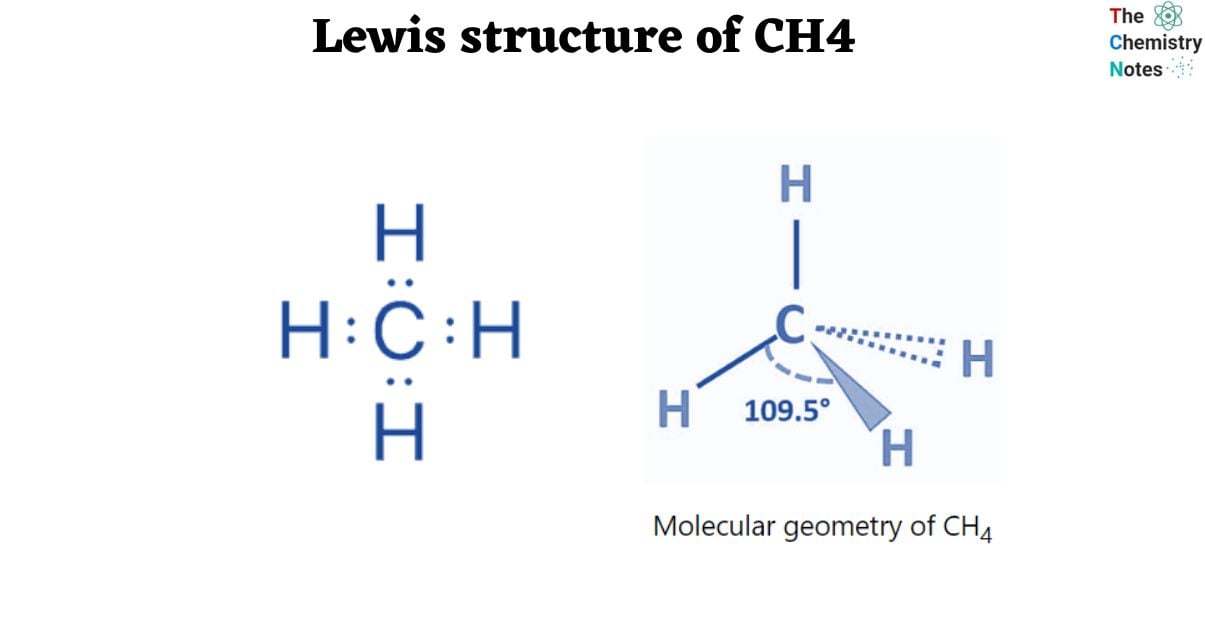
Lewis Structure of CH4
The Lewis structure is a highly simplified representation of the valence electrons in a chemical species such as an atom, ion, or molecule. It shows how electrons are arranged around atoms as lone pairs or bond pairs.
In the Lewis structure, dot do represents lone pairs of electrons and line represents bond pairs of electrons. The Lewis electronic/dot structure of molecules or ions is written using the octet rule.
Carbon, the central atom of methane, has four bound electron pairs. With four hydrogen atoms, it creates four equivalent covalent bonds. The four bonds are directed towards the four corners of a rectangular tetrahedron. According to VSEPR theory, the molecule has regular tetrahedron geometry with a bond angle of H-C-H of 109.5° since all four bond pairs are equivalent.
How to Draw Lewis Structure of CH4
Step 1: Count the number of valence electrons on each atom of the CH4 molecule.
As we know, carbon is an IVA group element with four elements in its last shell, but hydrogen is an IA group element with only one electron in its last shell, thus we now know the total valence electron in the valence shells of hydrogen and carbon atoms.
Valence electron by hydrogen = 4*1= 4
Valence electron by carbon = 1*4 = 4
As a result, total valence electrons = 8.
Step 2: Count the total number of electron pairs that exist as lone pairs and bonds.
Total valence electron pairs = σ bond + bonds + valence shell lone pairs
Total electron pair is calculated by dividing the total number of valence electrons by two.
Step 3: Select the central atom
Given that hydrogen has a maximum valence of one, carbon becomes the center atom and hydrogen cannot be a center atom.
Step 4: Indicate the lone pair on the atom
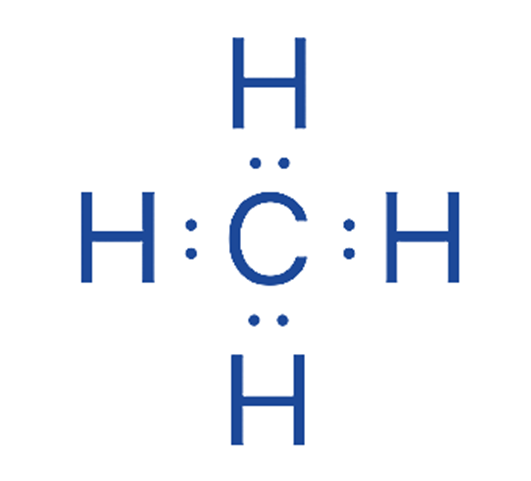
After locating the central atom and drawing the CH4 molecule, we may begin marking lone pairs on atoms. A total of four electron pairs must exist as lone pairs and bonds. C – H bonds are already present in the drawing below. As a result, there are no more electron pairs to label on atoms H.
Step 5: Label any charges that exist on atoms
As indicated in the structure, there are no charges on the carbon and hydrogen atoms.
Step 6: Determine the formal charge
Formal charge = Valence electrons (V) – Lone electron pair (L) – Bond electron pair (B)/2.
(formal charge)H = 1 – 0- (1/2)*2 = 0 This applies to each hydrogen, and because these hydrogens are all o, the molecule is neutral.
By converting lone pairs to bonds, we may test the stability of atoms and minimize their charges.
There are no charges on either the hydrogen or carbon atoms in the structure depicted above. As a result, we have derived the Lewis structure of CH4.
Molecular Geometry of CH4
The most basic of all organic substances is methane (CH4). It is made up of atoms of hydrogen (H) and carbon (C). Carbon is located in Group 14 and hydrogen is located in Group 1 of the periodic table. Consequently, there are four valence electrons in carbon and one in hydrogen. For carbon to complete its valence shell, four additional electrons are needed. To take on the electron configuration of its closest inert gas neighbor, helium, one electron is needed by hydrogen.
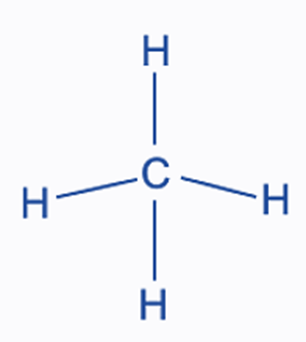
A molecule can be visually represented using dots and dashes to create a Lewis structure. The dash denotes a bond, and the dots stand for lone pairs. The CH4 molecule has four solitary C-H bonds since carbon does not have a lone pair.
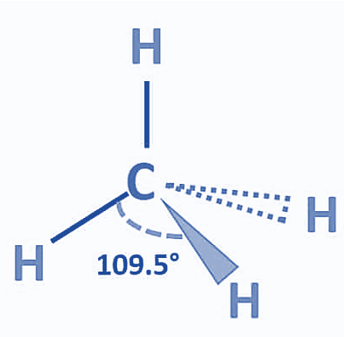
One useful method for determining the shape of molecules is the VSEPR theory. According to this theory, the bond electrons are positioned to have the least repulsive effect. A molecule without any lone pairs so exhibits a tetrahedral structural structure. The bond angle, or the angle formed by each individual C-H bond, is 109.5°. Methane’s tetrahedral structure is undistorted since it lacks lone pairs. The geometry of the electron and the molecules are the same. Methane has VSEPR notation AX4.
Video on Lewis Structure of CH4
References
- https://deskchem.com/ch4-lewis-structure/
- https://techiescientist.com/ch4-lewis-structure/
- https://www.sciencecoverage.com/2022/02/lewis-structure-of-ch4.html
- https://www.chemistrylearner.com/molecular-geometry/ch4-molecular-geometry
- https://lambdageeks.com/ch4-lewis-structure/

