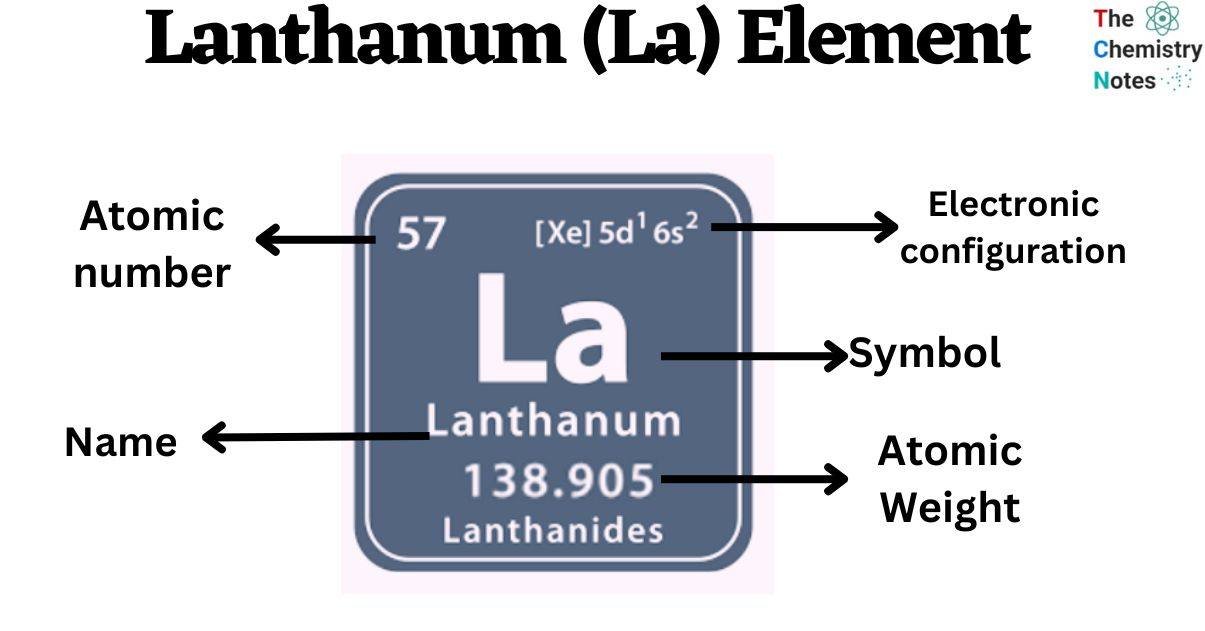
Lanthanum is a chemical element with the atomic number 57 and is represented by the symbol ‘La’ in the periodic table. It is soft and silvery in appearance and classified as rare earth metal and belongs to the d-block of the lanthanide group of the periodic table. It is a highly reactive rare earth metal only behind europium. Similar to the majority of other rare earth elements, the customary oxidation state of this particular element is +3. However, it is worth noting that certain compounds have been identified wherein the oxidation state is +2.
Lanthanum is the third-most prevalent lanthanide at 39 mg/kg of Earth’s crust. In the Earth’s crust, it is almost three times as prevalent as lead. Lanthanum is not rare, although it is rarer than lime and magnesia. Historically, only a limited number of deposits containing lanthanum were identified. Lanthanum is classified as a rare earth metal due to the arduous, protracted, and costly nature of its mining procedures.
Interesting Science Videos
History of Lanthanum
- The esteemed Swedish chemist Carl Gustaf Mosander is duly acknowledged for his momentous discovery of the element lanthanum.
- In the month of January in the year 1839, Carl Mosander, a diligent researcher at the esteemed Karolinska Institute in Sweden, successfully conducted an experiment involving a sample of cerium. Through his meticulous efforts, Mosander achieved the extraction of lanthanum, a notable accomplishment within the realm of scientific inquiry.
- The individual proceeded to subject powdered cerium nitrate to treatment with a solution of dilute nitric acid.
- He carefully observed the breakdown of a portion of the nitrate powder in the acid, prompting him to make the resolute choice of segregating the amalgamation from the resulting precipitate through the application of heat and sodium oxalate. The pallid brick-hued oxide procured was recognized with the label lanthanum.
- The given name of this chemical entity is derived from the ancient Greek term ‘lanthanein’, denoting the act of concealing or remaining obscured, owing to its protracted elusiveness within cerium oxide.
Occurrence of Lanthanum
- La is widely distributed throughout the Earth’s crust and does not exhibit localized occurrences comparable to copper or zinc. Lanthanum, an element of considerable abundance, is found in approximately 18 parts per million.
- La is commonly found in the ores of monazite, bastnasite, and cerite. In addition to the aforementioned minerals, it is noteworthy that the presence of other rare elements can also be observed.
- Even though there are only two isotopes of La that occur naturally, scientists have been able to synthesize more than a dozen radioactive isotopes by artificial means.
Isotopes of Lanthanum
There are two stable naturally occurring stable isotopes: 138La, and 139La.
Naturally Occurring Stable Isotopes of Lanthanum
| Isotopes | Natural abundance (atom %) |
|---|---|
| 138La | 0.090 (1) |
| 139La | 99.910 (1) |
Elemental Properties of Lanthanum
| Electronic Configuration | [Xe] 5d1 6s2 |
| Atomic Number | 57 |
| Atomic Weight | 138.90 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | no, 6, f-block |
| Density | 6.16 g.cm -3 at 20 °C |
| Key Isotopes | 139La |
| Van der Waals radius | 0.104 nm (+3) |
| Electron shells | 2, 8, 18, 18, 9, 2 |
| Electrons | 57 |
| Protons | 57 |
| Neutrons in most abundant isotope | 82 |
Physical Properties of Lanthanum
- Lanthanum has an atomic number of 57 and is a silvery-white rare earth metal. It has a melting point of 920 °C (1688 °F) and a boiling point of 3464 °C (6267 °F).
- La has a solid phase density of 6.162 g/cm3 and a liquid or molten phase density of 5.94 g/cm3.
- There is a gradual increase in hardness across the lanthanide series, with La being the softest.
- La is malleable which means it can be easily beaten into thin sheets without any cleavage.
- La is ductile which means it is possible to draw thin wires from it without breaking.
- In comparison to other metals within the lanthanide series, it exhibits a relatively modest degree of paramagnetism.
- It exhibits a hexagonal crystal structure when maintained at room temperature.
- At approximately 310°C, the crystalline structure of La changes to a face-centered cubic configuration. When the temperature is increased to approximately 865 °C, lanthanum’s crystal structure undergoes a second significant change, adopting a body-centered cubic configuration.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1193 K (920 °C, 1688 °F) |
| Boiling point | 3737 K (3464 °C, 6267 °F) |
| Density | 6.162 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.10 (Pauling Scale) |
Chemical properties of Lanthanum
- La exhibits the highest reactivity when compared to the other elements within the lanthanide series.
- La, among the lanthanides, exhibits the largest atomic radius. Consequently, it exhibits the highest level of reactivity within its group, undergoing rapid tarnish upon exposure to atmospheric conditions and assuming a completely darkened appearance within a matter of hours.
- At ambient conditions, the chemical element La exhibits reactivity with halogens, resulting in the formation of trihalides.
- La exhibits a propensity for undergoing oxidation upon interaction with water, resulting in the formation of its corresponding hydroxide.
- It dissolves quickly in diluted acids, with the exception of hydrofluoric acid (HF), and when it does so, it forms a layer of protective fluoride (LaF3) on the surface of the metal.
Chemical Reaction of Lanthanum
- The Reaction of Lanthanum With Water
La exhibits a gradual reactivity when exposed to cold water, whereas its reaction rate is significantly accelerated in the presence of hot water. This chemical process leads to the formation of lanthanum(III) hydroxide, denoted as La(OH)3, along with the liberation of hydrogen gas (H2).
2 La (s) + 6 H2O (g) → 2 La(OH)3 (aq) + 3 H2 (g)
- The Reaction of Lanthanum With Air
La undergoes gradual oxidation when exposed to air, leading to the formation of lanthanum (III) oxide, commonly known as La2O3.
4 La (s) + 3 O2 (g) → 2 La2O3 (s)
La exhibits a gradual oxidation process when exposed to atmospheric conditions, resulting in the formation of a thin layer of tarnish. Moreover, it displays a pronounced propensity to combust upon ignition, leading to the production of lanthanum(III) nitride (LaN) when subjected to a temperature of 450 °C.
2 La (s) + N2 (g) → 2 LaN (s)
- The Reaction of Lanthanum With Halogens
La metal exhibits reactivity with various halogens, resulting in the formation of lanthanum(III) halides.
The chemical reaction between La metal and fluorine gas (F2) results in the formation of lanthanum (III) fluoride (LaF3).
2 La (s) + 3 F2 (g) → 2 LaF3 (s)
The chemical reaction between la metal and chlorine gas (Cl2) results in the formation of lanthanum (III) chloride, denoted as LaCl3.
2 La (s) + 3 Cl2 (g) → 2 LaCl3 (s)
The chemical reaction between lanthanum metal and bromide, Br2, results in the formation of lanthanum (III) bromide, LABr3.
2 La (s) + 3 Br2 (g) → 2 LaBr3 (s)
The chemical reaction between La metal and iodine (I2) results in the formation of lanthanum (III) iodide, denoted as LaI3.
2 La (s) + 3 I2 (g) → 2 LaI3 (s)
- The Reaction of Lanthanum With Acids
La metal is easily dissolved in dilute sulphuric acid, which results in the formation of a solution that contains the aquated La(III) ion in addition to hydrogen gas, H2. There is a good chance that the complex ion [La(OH2)9]3+ makes up a significant portion of the La3+(aq) ion.
2 La (s) + 3 H2SO4 (aq) → 2 La3+ (aq) + 3 SO42- (aq) + 3 H2 (g)
Uses of Lanthanum
The utilization of lanthanum is observed in various domains.
It is classified as one of the elements belonging to the group known as rare earth elements. The chemical element in question is a malleable, pliable, lustrous, and silver-colored metal. The element La does not possess significant commercial applications; however, its alloys are widely utilized in various industries. Below, we will discuss several applications of the aforementioned subject.
Used In Batteries
La is extensively employed in significant quantities within nickel metal hydride rechargeable batteries designed for hybrid automobiles. Hybrid vehicles make use of a substantial quantity of lanthanum. The vehicles in question are equipped with batteries composed of nickel-lanthanum hydride. Furthermore, it should be noted that these batteries exhibit approximately double the efficiency when compared to conventional lead-acid car batteries.
Used In Glass And Ceramics
La finds utility in the fabrication of glass and ceramics. Special optical glasses, such as infrared absorbing glass, are manufactured utilizing this material. It is employed as a doping agent in camera and telescope lenses for its notable refractive value and favorable dispersion indices.
Used In Optical Industries
Lanthanum trifluoride is a crucial constituent within a heavy fluoride glass, renowned for its exceptional transmission capabilities. Consequently, it finds extensive application in the field of fiber optics communication.
Used In Drugs
Lanthanum carbonate is employed for the purpose of phosphate absorption, as observed in instances of hyperphosphatemia, such as those arising from end-stage kidney disease. La serves as an electron-dense tracer in the field of molecular biology.
Used In Electronics
La compounds are widely used in carbon arc lamps. The electrode gets heated by running a current of electricity through a carbon arc lamp. The electrode is constructed from carbon with small amounts of other components. As the carbon gets heated by electricity, it emits a bright white light. Depending on what else had been introduced to the carbon, the light that results might have any combination of colors. Energy-efficient light bulbs, televisions, fluorescent light bulbs, tubes, etc. also utilize the use of La’s chemical form.
Health Effects of Lanthanum
- La exhibits a relatively modest degree of toxicity, yet it necessitates careful handling and precautionary measures. The ingestion of lanthanum solutions has been observed to induce hyperglycemia, hepatic alterations, decreased blood pressure, and degeneration of the spleen.
- La, similar to other elements within the lanthanide series, has been observed to exert an influence on human metabolism. Additionally, it increases the likelihood of thrombosis, reduces blood pressure, alters cholesterol levels, and affects appetite.
Environmental Effects of Lanthanum
- La exhibits minimal toxicity, and its emissions resulting from coal combustion and mining activities are not considered to pose significant environmental hazards. Upon entering the atmosphere, la demonstrates an affinity for binding with carbonate and phosphate compounds.
- When phosphate is chemically bonded, it gives rise to the creation of the insoluble mineral known as Rhabdophane, which exhibits no harmful effects on the environment.
- Furthermore, it is of utmost importance for only a handful of bacterial species. Based on several studies, it has been observed that elevated levels of lanthanum, in conjunction with phosphate, have been associated with inhibited growth, delayed maturation, and reduced reproductive capacity in animals, ultimately leading to diminished population growth rates.
Video on Lanthanum Element
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1150–151. ISBN 978-0-08-037941-8.
- D. F. Shriver & P. W. Atkins, Inorganic Chemistry, 5th Edition, Oxford University Press, 2010.
- https://www.rsc.org/periodic-table/element/57/lanthanum
- https://www.britannica.com/science/lanthanum
- https://pubchem.ncbi.nlm.nih.gov/element/Lanthanum

