Introduction
Ammonia, an odorless gaseous compound, exhibits the property of colorlessness and is represented by the chemical formula NH3. The constitution of this entity consists of hydrogen and nitrogen. The term “ammonium hydroxide” is employed to denote its state when dissolved in water. The aforementioned inorganic compound demonstrates a remarkably discernible pungency in its odor. When present in a state of high concentration, it demonstrates inherent dangers and possesses corrosive characteristics.
The first recorded synthesis of ammonia is credited to Joseph Priestley in 1771. Priestley utilized a method involving the heating of ammonium and lime together. Subsequently, Priestley assigned the name “Alkaline Air” to this chemical. It plays a substantial role in meeting the nutritional needs of land-dwelling creatures, as it acts as a precursor to around 45% of the worldwide food supply and fertilizers. From a biological perspective, it is well noted that aquatic organisms frequently excrete nitrogenous waste material. Fertilizers with varying compositions, such as urea and diammonium phosphate, are made by utilizing around 70% of the ammonia produced. In addition, the practice of applying undiluted ammonia straight onto the surface of the soil is utilized.
Properties of Ammonia
| Molecular Formula | NH3 |
| Appearance, Odor | Colorless Gas, Strong and Pungent |
| Molar Mass | 17.031 g·mol−1 |
| Density | 0.73 kg/m³ |
| Boiling Point | -33.34 °C |
| Melting Point | −77.73 °C |
Preparation of Ammonia
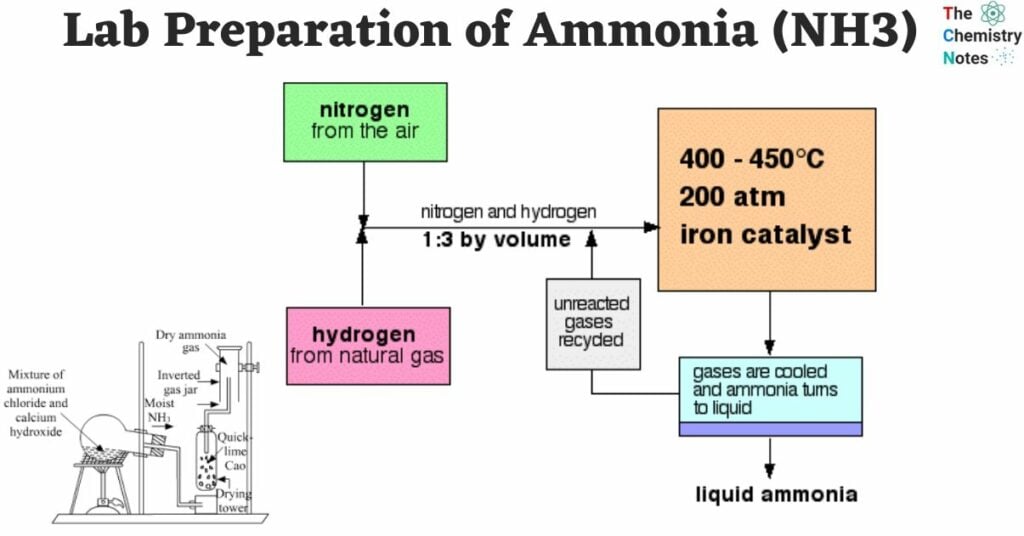
In the laboratory, ammonia can be readily produced by heating an ammonium salt, such as ammonium chloride (NH4Cl), with a strong alkali like sodium hydroxide or calcium hydroxide.
2 NH4Cl + Ca(OH)2 → CaCl2 + 2 H2O + 2 NH3 (g)The production of gas can also be achieved by heating concentrated ammonium hydroxide.
The Haber Process is the primary commercial method used to produce ammonia. It involves the direct combination of nitrogen and hydrogen in the presence of a catalyst while being subjected to high pressure.
Manufacturing Ammonia Using Haber’s Process
Ammonia is synthesized on an industrial scale using three main processes: Haber’s Process, Cyanamide’s Process, and Serpeck’s Process. The Haber process is known for being a cost-effective method of synthesis.
- Dr. Fritz Haber, a German chemist, and Nobel Laureate, made a significant contribution to the field of chemistry by developing an efficient method for producing ammonia. This Method involves directly combining hydrogen and nitrogen gas under specific conditions, resulting in the economical production of ammonia.
- Dr. Haber’s groundbreaking work in this area has had a lasting impact on the field of chemistry. He was born in Breslau, Germany, which is now known as Wroclaw, Poland. He received his education at the Technische Hochschule in Berlin. After completing his studies in chemistry, he was granted the prestigious position of Professor of Physical Chemistry at the University of Berlin in 1911.
- In recognition of his groundbreaking work in the development of synthetic ammonia, fertilizers, and explosives, he was honored with the Nobel Prize in chemistry in 1918. He is widely recognized as “The Father of Chemical Warfare” due to his significant contributions to the development and deployment of chlorine and poison gases during World War I. The German chemist Karl Bosch introduced the method for commercial use in the 1930s.
Raw Materials
The production of ammonia entails the utilization of different materials. The utilization of these primary constituents is imperative for the synthesis of ammonia.
- Nitrogen
- Hydrogen
- The utilization of Al2O3 and K2O as promoters for iron and
- Zirconium dioxide (ZrO2) as catalyst promoter.
Principle
Under normal circumstances, nitrogen, which is an inert gas, does not react or combine with hydrogen.
- Ammonia can be produced by heating a mixture of dry nitrogen and hydrogen.
- When measuring by volume, the ideal volume ratio for this mixture is 1:3.
- The reaction takes place in the presence of a catalyst, typically promoted iron, which contains small amounts of metal oxides like Al2O3, K2O, and ZrO.
- The reaction occurs at a temperature range of approximately 450°C to 500°C and a pressure range of 200 to 900 atm.
N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g) + 22 Kcal
1 vol. 3 vol. 2 vol.Conditions For High Yield of Ammonia
Given the aforementioned synthetic reaction’s reversible nature, exothermic behavior, and tendency to occur with a reduction in volume, it is anticipated that Le Chatelier’s principle would indicate the following advantageous circumstances:
Low Temperature
- Based on the exothermic nature of the equilibrium reaction, it can be deduced, in accordance with Le-Chatelier’s principle, that lower temperatures are favorable for the production of ammonia.
- In the realm of industrial ammonia production, the feasibility of relying on the reaction between Nitrogen gas (N2) and Hydrogen gas (H2) at temperatures below 450 °C is considered unviable due to its inherent sluggishness.
- As a result, a thermal condition within the range of approximately 450 °C to 500 °C is implemented.
High Pressure
- The equilibrium reaction involving the gaseous compounds hydrogen (H2) and nitrogen (N2) exhibits a phenomenon wherein the volume of the resulting product experiences a reduction.
- An increase in pressure, ranging from approximately 200 to 900 atmospheres, promotes the preferential synthesis of ammonia.
High Concentration
- The reaction proceeds in the forward direction as the volume diminishes.
- To optimize the synthesis of ammonia (NH3), it is recommended to utilize a surplus of either or both of the reactants, specifically hydrogen (H2) or nitrogen (N2).
Catalyst
- The incorporation of a catalyst serves to accelerate the rate of the chemical reaction kinetics.
- It is highly recommended to employ finely dispersed iron, accompanied by aluminum oxide (Al2O3), potassium oxide (K2O), and zirconium dioxide (ZrO2) as a promoter, in order to enhance the overall performance.
Purity of Hydrogen and Nitrogen
- In order to maintain optimal catalytic activity, it is crucial to ensure that the nitrogen and hydrogen gases used are of high purity.
- If these gases are contaminated, the catalyst can become poisoned, leading to a decrease in its effectiveness.
Details of the Process
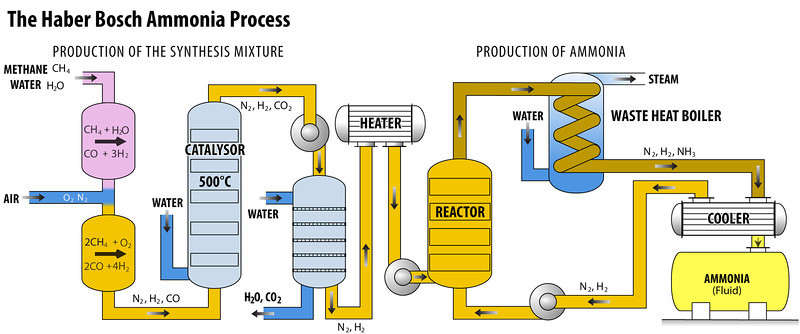
Production of Hydrogen and Nitrogen
- Hydrogen gas can be obtained through two methods: electrolysis of water or the formation of water gas (CO + H2).
- In the first method, water is subjected to electrolysis to separate hydrogen and oxygen.
- The second method involves combining carbon monoxide (CO) and hydrogen (H2) to produce water gas, and then fractional liquefaction to remove the carbon monoxide.
- Nitrogen is obtained through a process called fractional distillation of liquid air.
Compressor
- The gaseous elements nitrogen and hydrogen, in a volumetric ratio of 1:3, undergo compression within the purifying unit.
- This compression process is facilitated by a compressor pump, which operates at pressures ranging from 200 to 900 atmospheres.
Purifying Unit
- The compressed gas is subsequently directed into a soda-lime tower, which consists of a combination of sodium hydroxide and calcium oxide, commonly referred to as soda-lime.
- This material has the ability to absorb carbon dioxide (CO2) and moisture from its surroundings.
2 NaOH + CO2 → Na2CO3 + H2OCaO + H2O → Ca(OH)2Catalyst Chamber
- A vertical, cylindrical steel vessel with thick walls is designed to withstand high gaseous pressure.
- The material is composed of finely divided iron that has been promoted with Al2O3, K2O, and ZrO.
- Carl Bosch created the equipment and catalyst choices for Haber’s process, which led to the creation of the commonly known Haber-Bosch ammonia synthesis.
- The chamber is electrically heated to approximately 500 °C in order to initiate the reaction. The reaction is exothermic, meaning that it releases heat as a byproduct.
- This heat generated by the reaction helps sustain and continue the reaction.
- At the given temperature and pressure, it is observed that approximately 15% of the reactant gases undergo conversion to form ammonia.
Condenser
- The gas that is obtained from the catalyst chamber consists of ammonia as well as unreacted hydrogen and nitrogen. After the mixture is processed, it is directed through a condenser.
- In this condenser, ammonia undergoes condensation and is gathered in a receiver.
- On the other hand, any unreacted hydrogen (H2) and nitrogen (N2) remain in their gaseous states and do not condense.
- Liquor ammonia is the term used to refer to ammonia that has been condensed.
Recirculation
- A recirculation pump is an essential component used to circulate the uncondensed gas, which primarily consists of unreacted nitrogen and hydrogen, through the catalyst converter.
- This process enables the gas to undergo reprocessing, resulting in the production of additional ammonia.
Chemical Properties of Ammonia
Ammonia has certain chemical properties:
- Because of its fundamental composition, ammonia is highly soluble in water. The weakly basic nature of its aqueous solution can be attributed to the presence of OH- ions generated during its production.
NH3(g)+H2O(I) ⇋ NH4OH(aq) ⇋ NH4+(aq)+OH–(aq)
Sodium hydroxide exhibits basic properties. When in contact with moist red litmus paper, it causes a color change from red to blue, indicating its alkaline nature. Additionally, sodium hydroxide has the ability to neutralize acids in both dry and wet conditions, resulting in the formation of salts specific to the acid being neutralized.
NH3+HCl → NH4Cl
2NH4OH+H2SO4 → (NH4)2SO4+2H2O
- Ammonia is classified as a Lewis base because it possesses a lone pair of electrons on its nitrogen atom. As a result, the molecule has the ability to donate its electron pair, leading to the formation of a coordinate bond. This bond is established with electron-deficient compounds like BF3 or transition metal cations that have unoccupied d-orbitals. The outcome of this interaction is the formation of complexes.
Ag+(aq)+2NH3(aq) → [Ag(NH3)2]+(aq)
Cu2+(aq)+4NH3(aq) → [Cu(NH3)4]2+(aq)
- Ammonia does not function as a fuel or a combustion enhancer. Under aerobic conditions, the reaction of this substance yields dinitrogen and water as the resulting products.
4NH3+3O2→2 N2+6H2O
- The oxidation of ammonia to dinitrogen gas can be achieved by passing it through various substances, such as a solution of calcium hypochlorite (commonly known as bleaching powder), bromine water, or heated copper oxide.
4NH3+3Ca(OCl)2 → 2 N2+3CaCl2+6H2O
8NH3+3Br2 → N2+6NH4Br
2NH3+3CuO+Heat → 3Cu+N2+3H2O
Applications of Ammonia
- Ammonia is commonly used as a fertilizer due to its ability to enhance crop yield.
- It is commonly used as a household cleaner. It is a compound with the chemical formula NH3. When mixed with water, it becomes an effective solution for cleaning stainless steel and glass surfaces.
- It is commonly utilized in food products as an antimicrobial agent.
- It plays a crucial role in the fermentation industry.
- It is commonly employed as a refrigerant.
- It is commonly employed as a pH adjuster during the fermentation process.
- It is commonly employed as a means to neutralize pollutants, such as nitrogen oxides, that are emitted from diesel engines.
- It serves as a fuel for rocket engines.
References
- https://byjus.com/chemistry/ammonia/#:~:text=Preparation%20of%20Ammonia%20%E2%80%93%20NH&text=Ammonia%20is%20easily%20made%20in,sodium%20hydroxide%20or%20calcium%20hydroxide.
- https://srjng88.medium.com/ammonia-gas-preparation-properties-uses-7f37e1db23a1
- https://gkscientist.com/preparation-and-manufacture-of-ammonia/
- https://unacademy.com/content/neet-ug/study-material/chemistry/the-preparation-of-ammonia/
- https://www.geeksforgeeks.org/ammonia-structure-properties-preparation-uses/
- https://collegedunia.com/exams/ammonia-chemistry-articleid-2978
- https://chemicalnote.com/ammonia-manufacture-by-habers-process-properties-uses-and-structure/
