A type of addition reaction called the Kolbe reaction is named after Hermann Kolbe and Rudolf Schmitt. It is also known as the Kolbe-Schmitt Reaction or Carboxylation reaction. The Kolbe-Schmitt reaction involves the utilization of carbon dioxide gas, a base, and acid to transform phenol into hydroxy-benzoic acid.
Hermann Kolbe and Rudolf Schmitt discovered and named this reaction. Hermann Kolbe played a crucial role in developing modern organic chemistry.
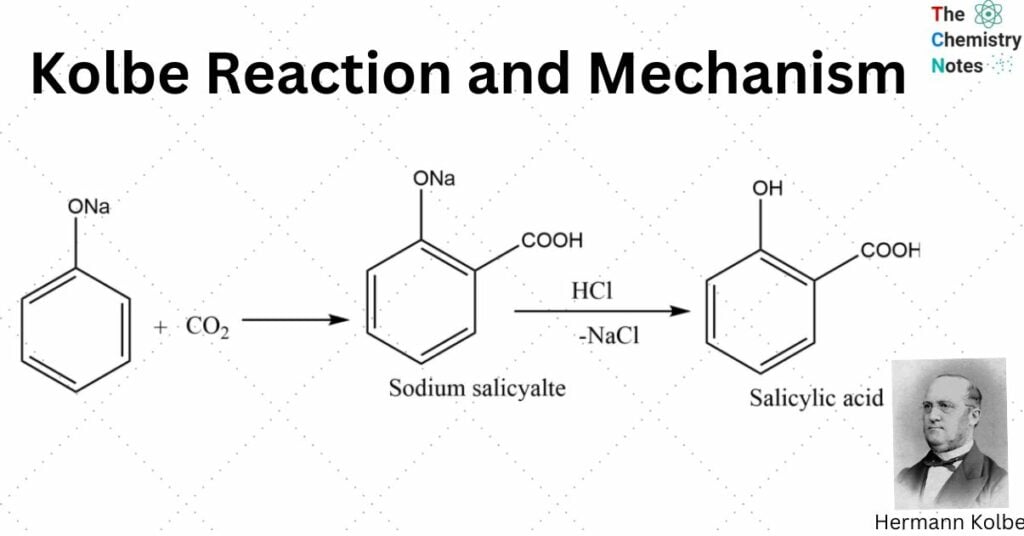
What is Kolbe Reaction?
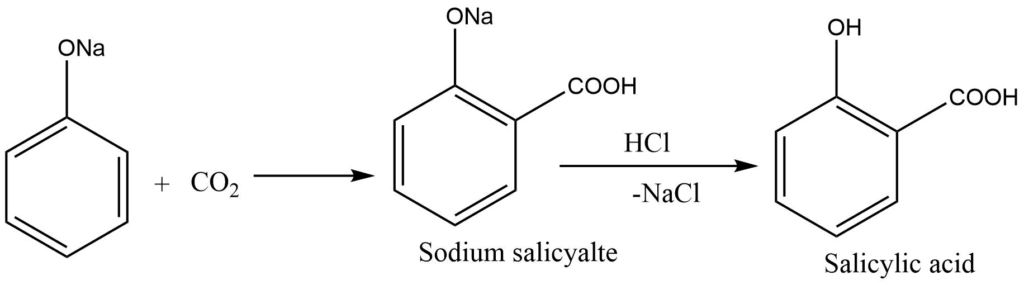
The process by which sodium phenoxide is converted into sodium salicylate by heating it with carbon dioxide at temperatures between (120 -140) degrees Celsius under pressure is known as Kolbe’s reaction.
When it comes to an electrophilic aromatic substitution process, the produced phenoxide ion is more reactive than phenol. The mild electrophilicity of carbon dioxide causes it to perform an electrophilic substitution reaction with the molecule. The main byproduct is ortho-hydroxybenzoic acid, also known as salicylic acid. Kolbe’s response is the common name for this phenomenon.
Potassium hydroxide allows for the production of 4-hydroxybenzoic acid, a key precursor to the broad-spectrum biocide family known as parabens, which is found in many common consumer goods.
3-hydroxy-2-naphthoic acid is synthesized using the same process; the regiochemistry of the carboxylation in this case is temperature-dependent.
Kolbe’s Reaction Mechanism
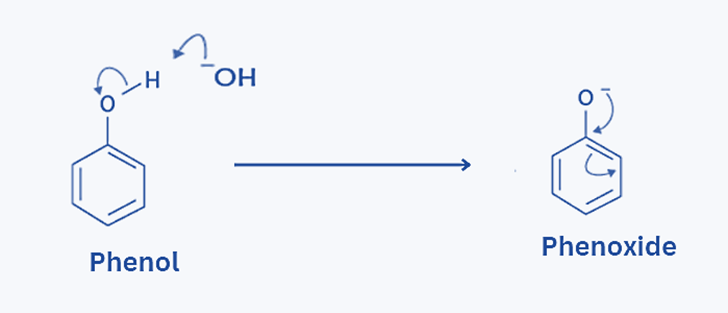
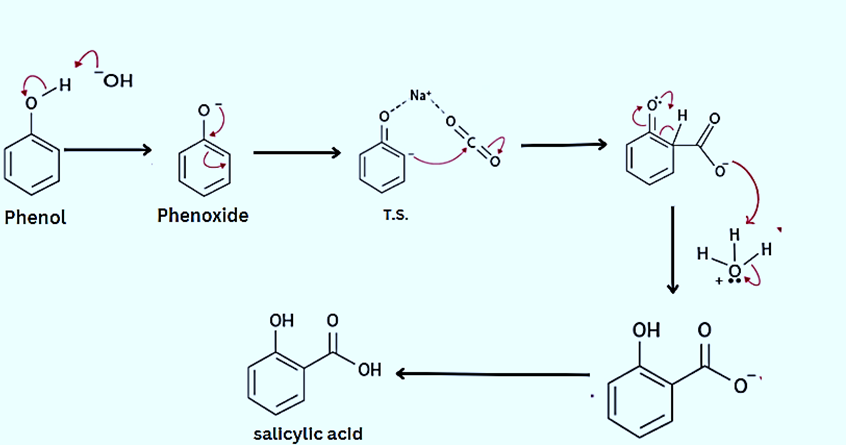
Step 1: Phenol and sodium hydroxide combine to produce phenoxide ion
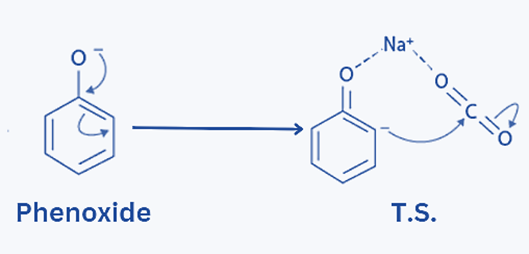
Step 2: By forming a transition state with Na+ and CO2, the negative phenoxide ion moves to the ortho position.
Step 3: The proton moves from the ortho position to the Oxygen bonded to the benzene ring when the -ve ion attacks the Carbon of CO2.
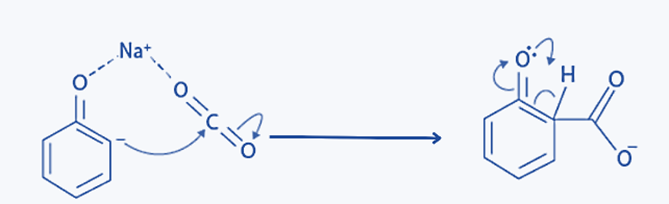
Step 4: The carboxylate ion is attacked by H+/H3O+ (from any mineral acid like H2SO4) because of the proton shift. As a result, salicylic acid/ortho-hydroxy benzoic acid is produced, with water being a by-product of the reaction.
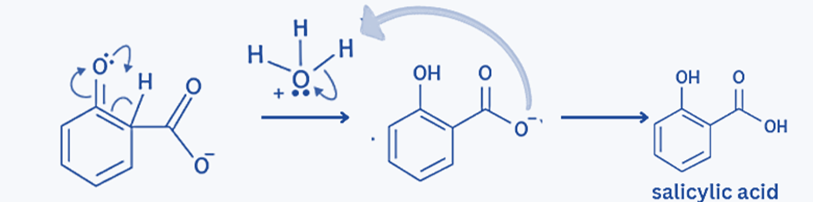
Overall Mechanism of Reaction is:
Application of Kolbe Reaction
- Aspirin is derived from salicylic acid by esterification (in this case, a reaction with acetic anhydride).
- Aspirin is a common analgesic used in modern medicine.
- In order to create various pigments and colours, salicylic acid is converted into 3-hydroxy 2-naphthoic acid.
- In addition to benzoic acid, salicylic acid is also utilized to make 4-hydroxybenzoic acid.
- Sodium acetate may be converted to ethane via the Kolbe reaction. An aqueous sodium acetate solution is electrolyzed using the Kolbe procedure. Methyl radicals are produced as a result of the decomposition of acetate ions. They react with other free methyl radicals to produce ethane.
- It’s a crucial ingredient in making cosmetics and personal care items.
Historical Facts of Kolbe Method
- In 1860, when Kolbe heated a solution of phenol and sodium in the presence of carbon dioxide at room temperature and atmospheric pressure, he produced salicylic acid. Salicylic acid was precipitated from a solution of sodium salicylate in water by adding acid. Kolbe wanted to demonstrate that salicylic acid was monobasic, therefore he synthesized it from phenol and carbon dioxide.
- Several unsuccessful attempts to produce salicylic acid in this manner led to the successful synthesis in 1860. From p-cresol and thymol, respectively, Kolbe also synthesized p-cresotinic acid and o-thymotinic acid using the same method.
- Several years later, Kolbe needed to make a big batch of salicylic acid. The yield of salicylic acid, however, was observed to vary substantially under circumstances that seemed to be identical to those used in the initial synthesis.
- Kolbe proposed a novel method for producing salicylic acid after considering these findings. As half of the initial phenol was lost to volatilization, the maximum yield of salicylic acid obtained using this method never exceeded 50%.
- In 1884, Schmitt proposed a method for producing salicylic and homologous acids as well as hydroxy naphthoic acids by modifying the Kolbe reaction by performing the carbonation under pressure, which considerably increased the yields. The processes were refined later on. Dry sodium phenoxide, for instance, is heated in a closed tank with carbon dioxide at 120-130 degrees Celsius for many hours at a pressure of 80-94 atmospheres. No phenol is wasted and the production of salicylic acid is nearly quantifiable under these circumstances.
Kolbe-Schmitt reaction, a modified version of the original approach, is still widely used to produce a wide range of aromatic hydroxy acids.
References
- A. S. Lindsey and H. Jeskey (1957). “The Kolbe-Schmitt Reaction”. Chem. Rev. 57 (4): 583–620. doi:10.1021/cr50016a001.
- R. T. Morrison and R. N. Boyd (1983). Organic Chemistry (4th ed.). Allyn and Bacon.
- Gerald Booth (2005). “Naphthalene Derivatives”. Ullmann’s Encyclopedia of
Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_009 (http
s://doi.org/10.1002%2F14356007.a17_009).. - https://protonstalk.com/alcohols-phenols-ethers/kolbes-reaction/
- THE KOLBE-SCHMITT REACTION Alan S. Lindsey Harold Jeskey, https://doi.org/10.1021/cr50016a001
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
