Isomerism occurs when many substances have the same chemical formula but different chemical structures. The term “isomer” comes from the Greek words “isos” and “meros,” both of which signify “equal parts.” In the year 1830, the Swedish chemist Jacob Berzelius coined this term. Isomerism results from the fact that the atoms in a molecular formula can be organized in several ways to produce compounds known as isomers.
Isomers are molecules having the same molecular formula but various atom configurations, and the phenomenon is therefore called Isomerism. Isomers differ in their physical and chemical properties. Isomerism occurs when many substances have the same chemical formula but different chemical structures.
Isomerism refers to the property of two or more compounds that have the same molecular formula but differing physical and chemical properties.
Interesting Science Videos
Types of isomerism
Isomerism is classified into two types: structural isomerism (constitutional isomerism) and stereoisomerism. These are further subdivided into many subtypes.
Constitutional isomerism
Constitutional isomerism is also known as structural isomerism. The functional groups and atoms in these isomers’ molecules are linked in various ways. Since structural isomers may or may not include the same functional group, they are given different IUPAC designations.
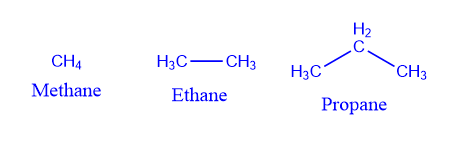
The simplest hydrocarbons, methane (CH4), ethane (CH3CH3), and propane (CH3CH2CH3), have no constitutional isomers because there is no other method to connect the carbons and hydrogens of these molecules that are consistent with carbon’s tetravalency and hydrogen’s univalency. The number of possible constitutional isomers increases exponentially with the number of atoms available.
Types of constitutional isomers
Chain isomerism
It is also known as skeletal isomerism. These isomers’ components have a variety of branching structures. Carbon branching differs amongst chain isomers.
Chain isomers are isomeric compounds that differ only in the arrangement of carbon atoms in the base chain, and chain isomerism is the isomeric relationship between them. The carbon atom chain or skeleton varies between this type of isomer.
Chain isomerism in alkanes:
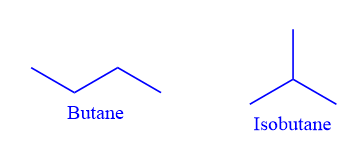
Alkanes with four or more carbon atoms exhibit chain isomerism. For example, the chemical formula C4H10 represents two-chain isomers. However, there are two distinct butanes, C4H10, and these two molecules, known as butane and isobutane, are constitutional isomers. They are several molecules with various chemical and physical properties. Butane is made up of four carbon atoms that are linked together in a continuous chain. Isobutane consists of a branched structure.
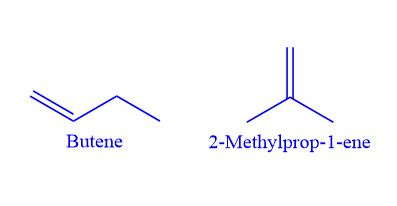
Chain isomerism in alkenes:
The position of the double bond should not be changed when writing chain isomers for alkenes. For example, the chemical formula C4H8 represents two-chain isomers.
Chain isomerism in alkynes:
Alkynes, which have five or more carbon atoms, exhibit chain isomerism. The chemical formula C5H8 represents the two chain isomers.
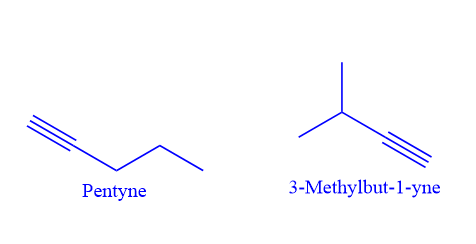
Positional isomerism
In position isomerism, isomers have identical functional groups but are located at different positions on the same carbon chain. This isomerism is typically caused by the binding of functional groups to distinct carbon atoms in the carbon chain. Positional isomer consist of ,
(i) The same molecular formula
(ii) The same carbon chain length
(iii) The same functional group
Position isomerism also exists in di-substituted derivatives due to the relative positions of the substituents on the benzene ring.
An example is the compound with molecular formula C6H4Br2, of which there are three isomers: 1,2-dibromobenzene, 1,3-dibromobenzene and 1,4-dibromobenzene. These isomers differ in the. position of substituent.
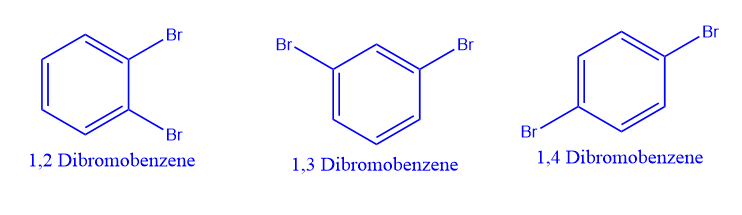
Functional isomerism
When isomers have the same chemical formula but distinct functional groups, they are referred to as functional group isomers, and the process is referred to as functional group isomerism.
The substance with the molecular formula C2H6O, for example, has two isomers: dimethyl ether and ethanol or ethyl alcohol, which have different functional groups, an ether group, -O-, and a hydroxyl group, -OH.
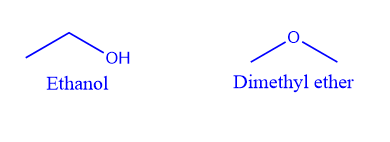
Metamerism
This type of isomerism results from the relative position of alkyl groups surrounding polyvalent functional groups (such as S, N, O, and CO) in a molecule at different locations. Metamerism occurs as a result of the presence of distinct alkyl chains on each side of the functional group.
(i) Diethyl ether and methyl propyl ether
CH3 CH2 O CH2 CH3 Diethyl ether, CH3 OCH2 CH2 CH3 Methyl propyl ether
(ii) Diethyl amine and methyl propylamine
CH3 CH2 –NH– CH2 CH3 Diethyl amine, CH3 CH2 CH2 –NH– CH3 Methyl propyl amine
Tautomerism
Tautomerism is the dynamic equilibrium of two molecules that have the same chemical formula. A tautomer of a compound is an isomer of the compound that differs solely in the location of protons and electrons. Typically, the tautomers of a molecule coexist in equilibrium and are easily interchangeable. It happens as a result of an intramolecular proton transfer. Keto-enol tautomerism is the most prevalent type of tautomerism. The transfer of a -hydrogen onto the oxygen atom converts a carbonyl molecule containing at least one α-hydrogen atom to an enol.
For example,
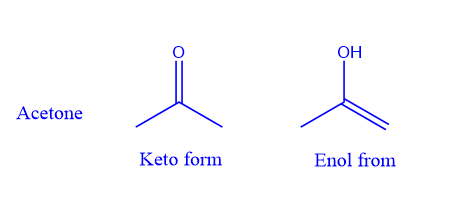
Tautomers are functional isomers that coexist in dynamic equilibrium.
Ring chain isomerism
Ring chain isomers are compounds that have the same chemical formula but have open-chain or cyclic structures. The phenomenon is known as ring-chain isomerism.
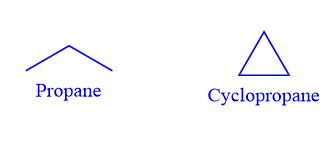
Stereoisomerism
Stereoisomerism is the term used to describe the phenomena of stereoisomers, which are molecules with the same chemical formula but differing atom spatial arrangements.
Conformational isomerism
Stereoisomers in conformational isomerism can be interconverted by rotating around one or more single bonds, the bonds. These rotations result in non-superimposable configurations of atoms in space.
Staggered conformation
Staggered conformation is the arrangement of atoms or groups of atoms in a molecule that results in a dihedral angle of a 60o. The atoms or groups of atoms are arranged in this conformation to reduce the repulsions between the bond electron pairs, which results in a low strain in the molecule.
Eclipsed conformation:
The arrangement of atoms or groups of atoms in a molecule that results in a dihedral angle of 0o is known as eclipsed conformation. Consequently, this conformation has relatively low stability. There is a strong repulsion between the bond electron pairs in the eclipsed conformation because there are no spaces between the atoms or groups of atoms. As a result, the eclipsed conformation is less stable.
For example, consider ethane, C2H4, and observe the molecule from one end down the carbon-carbon link using the Newman projection, hydrogen atoms of one methyl group can be in one of the following conformations with regard to the hydrogen atoms of the other methyl group.
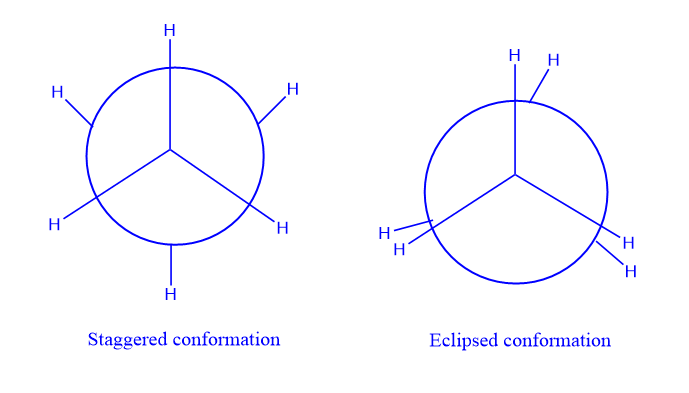
Configurational isomerism
In configurational isomerism, the interconversion between the stereoisomers does not occur as a result of rotations around single bonds but involves bond breaking and new bond forming, then it doesn’t occur spontaneously at room temperature.
Geometrical isomerism
Geometrical isomerism, also known as cis-trans isomerism, occurs when atoms are unable to freely rotate due to a rigid structure, such as those observed in • compounds with carbon-carbon, carbon-nitrogen, or nitrogen-nitrogen double bonds, where the rigidity is due to the double bond; and cyclic compounds, where the rigidity is due to the ring structure.
The double bond between the two carbon atoms is thus responsible for the geometric isomerism. Geometrical isomers are isomers that have the same chemical formula but differ in the spatial arrangement of groups or atoms and do not exhibit optical activity.
Carbon and carbon double-bond compounds are planar. As a result, if a molecule has two different atoms or groups attached to the carbon atom, it will form two isomers due to the different spatial arrangements of the groups. As a result, geometrical isomers are classified as cis and trans isomers.
Cis isomer
Cis isomer specifies the orientation when the two substituents with the highest priority are on the same side of the double bond.
Trans isomer
Trans isomer specifies the orientation when the two substituents with the highest priority are on the same side of the double bond.
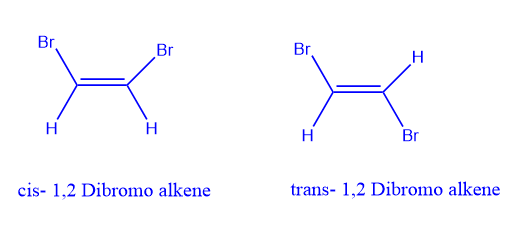
Optical isomerism
Optical isomerism occurs in molecules with one or more chirality centers or chiral centers, particularly tetrahedral atoms with four distinct ligands.
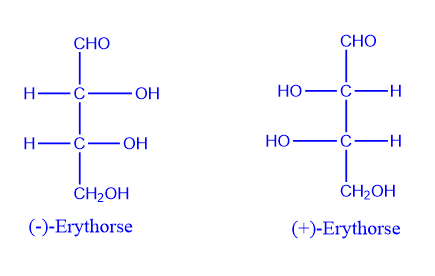
Most of their chemical interactions are likewise similar. One rotates plane polarized light to the right, while the other rotates it to the left, but to the same degree. This is the one case in which they behave differently. An optical isomer is a chemical pair with identical optical characteristics. Optical isomers are two compounds that have the same number and type of atoms, bonds, and spatial arrangements of the atoms but are not superimposable mirror images of each other.
Optical isomers are mirror images of one another and cannot be superimposed on one another because they lack a center of symmetry or a plane of symmetry. Enantiomers are named after the Greek words enántios, which means “opposite,” and meros, which means “part.”
Optical isomerism’s name comes from the direction in which they rotate the plane of polarized light rotates.
When one enantiomer solution rotates the plane of polarized light clockwise, the enantiomer is called (+). If the enantiomer rotates the plane of polarized light counterclockwise by the same angle, and the enantiomer is denoted by (-).
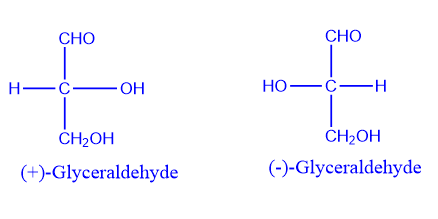
Enantiomers, unlike other isomers, are identical in terms of their physical and chemical characteristics.
Diastereomers are non-identical, non-mirror image stereoisomers. As a result, they occur when two or more stereoisomers of the same substance are not mirror images and have distinct configurations at one or more of the equivalent stereocenters. They can be optically active or inactive. The optically active diastereomers do not rotate plane-polarized light in opposite directions to an equal extent. They have different chemical and physical properties.
References
- K.R palak,2017, Stereochemistry. Pairavi Prakashan.
- Smith M. B. & March J. (2007). March’s advanced organic chemistry : reactions mechanisms and structure (6. ed.). Wiley-Interscience.
- Bahl A. & Bahl B. S. (2006). A textbook of organic chemistry (for b. sc. students) (18th rev. & enlarged ed. 1st multicolour illustrative). S. Chand.
- https://www.britannica.com/science/isomerism/Chirality-in-natural-and-synthetic-materials
- https://byjus.com/chemistry/isomerism/#:~:text=Isomerism%20is%20the%20phenomenon%20in,the%20molecule%20are%20called%20isomers.
- https://www.vedantu.com/chemistry/isomer.
