The term “isoelectronic” is used to describe two atoms, ions, or molecules that share the same electronic structure and have an equal number of valence electrons. The term signifies “equal charge” or “equal electric”.
Isoelectronic species are atoms, molecules, or ions that have the same number of electrons.
Isoelectronic species are chemical species that have the same number of electrons. These species often exhibit similar chemical properties. Atoms or ions that have the same electronic configurations are referred to as isoelectronic with each other.
Interesting Science Videos
Isoelectronic Ions
Isoelectronic ions are ions that have the same number of electrons.
One example of an isoelectronic ion is the K+ ion, which is isoelectronic with the Ca2+ ion. Carbon monoxide (CO) is isoelectronic with nitrogen (N2) and NO+.
Isoelectronic Molecules
Isoelectronic molecules are groups of two or more atoms or molecules that have identical electronic configurations and structures but differ in the specific elements present within their structures.
Examples of isoelectronic molecules include carbon monoxide (CO), nitric oxide cation (NO+), and dinitrogen (N2).
Isoelectronic Series
An isoelectronic series is formed by atoms, ions, or molecules that have an equal number of electrons.
Examples of an isoelectronic series include nitrogen (N2), carbon monoxide (CO), and oxygen cation (O+).
O2−, F−, Na+, Mg2+, and Al3+ all have different numbers of electrons.
Another example
N3–, Al3+ and Mg2+ have different numbers of electrons. N3- has 10 electrons, Al3+ has 10 electrons, and Mg2+ has 12 electrons.
He, H–, and Li+ are isoelectronic species because they all have the same number of electrons, which is 2. This means they also have the same electronic configuration.
It is important to mention that all isoelectronic species exhibit the same chemical properties.
Characteristics of Isoelectronic Species
- These species differ in terms of their atomic numbers and atomic masses, which is the sum of protons and neutrons. However, they have the same number of electrons.
- Isoelectronic species are atoms, ions, or molecules that have the same number of electrons. This means that they also have the same electronic configuration or arrangement of electrons in their energy levels.
- Elements with the same electronic configuration tend to have similar values for electronegativity, ionization energy, and chemical reactivity.
- The atomic radius of isoelectronic species can vary.
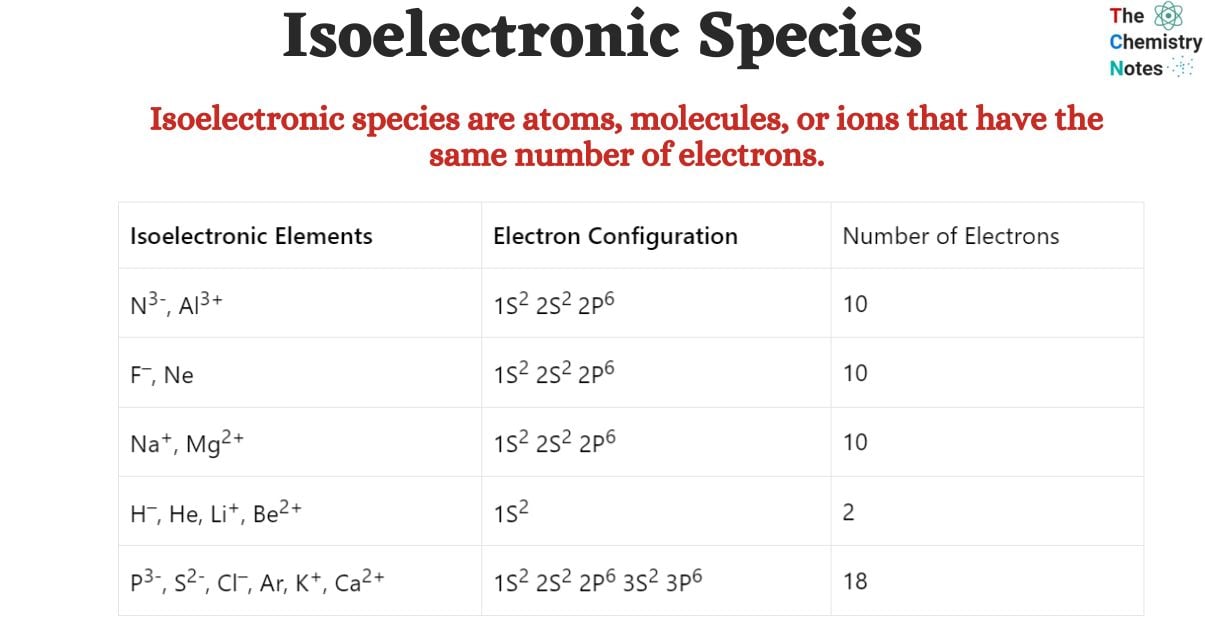
Isoelectronic Elements and Their Electron Configuration
| Isoelectronic Elements | Electron Configuration | Number of Electrons |
| N3-, Al3+ | 1S2 2S2 2P6 | 10 |
| F–, Ne | 1S2 2S2 2P6 | 10 |
| Na+, Mg2+ | 1S2 2S2 2P6 | 10 |
| H–, He, Li+, Be2+ | 1S2 | 2 |
| P3-, S2-, Cl–, Ar, K+, Ca2+ | 1S2 2S2 2P6 3S2 3P6 | 18 |
References
- Smith, Michael, and Jerry March. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. Hoboken, NJ: Wiley-Interscience, 2007.
- Rayner-Canham, Geoff. “Isoelectronic Series: a Fundamental Periodic Property.” Foundations of Chemistry 11.2 (2009): 123-29.
- https://chemistnotes.com/inorganic/isoelectronic-species-easy-definition/
- https://www.vedantu.com/iit-jee/isoelectronic-definition
- Helmenstine, Anne Marie, Ph.D. “Isoelectronic Definition.” ThoughtCo, Aug. 28, 2020, thoughtco.com/definition-of-isoelectronic-605269.
