Isocyanides, also known as Isonitriles or Carbylamines, are any of an organic compound with the molecular formula R – N ≡ C, where R is a combining group derived by the ejection of a hydrogen atom from an organic compound. So, Isocyanide is a chemical compound with the functional group NC. It is a colorless, volatile poisonous liquid with a strong odor. Isocyanide is used in the manufacture of polyurethanes, as well as medicines, dyes, and other compounds. They are susceptible to polymerization and are isoelectronic with CO in terms of bonding.
They are nitrile isomers that were discovered in 1867. They are typically made from primary amines by treating them with chloroform and an alkaline solution, and they are frequently produced as byproducts during the synthesis of nitriles from metal cyanides and organic halogen compounds. They have highly potent and unpleasant scents.
Isocyanides are a class of reagents that are extremely versatile and useful because the isocyano group can act as both a nucleophile and an electrophile in chemical processes. Isocyanide chemistry has received a lot of attention and has found a number of uses in synthetic organic and medicinal chemistry for the creation of structurally fascinating and diverse N-heterocycles or peptidomimetics over the last few decades.
Interesting Science Videos
Structure of isocyanides
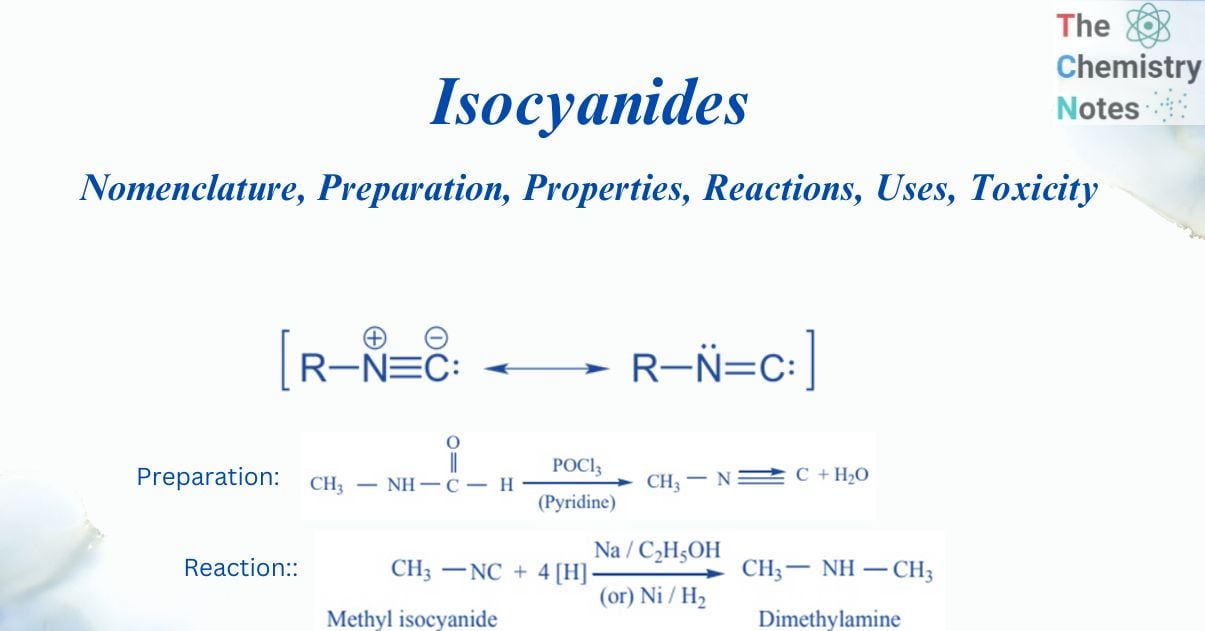
An isocyanide is an organic molecule containing a carbon-nitrogen triple bond and an alkyl or aryl group attached to the nitrogen. They have two resonance structures: one with a triple bond between carbon and nitrogen and one with a double bond between carbon and nitrogen. Although tests demonstrate that the structure with the carbon-nitrogen double bond contributes more to the resonance, the second structure is also required due to the linearity of the carbon-nitrogen bond angle (which is close to 180 degrees). Although the reactivity of isocyanides reflects certain carbene features, the π single pair of nitrogen sets the design and is capable of linearity. Both resonance structures are useful representations in this way. They are polymerization susceptible.
Nomenclature of isocyanides
Common name system:
In this system, cyanides are named either alkyl isocyanides or aryl isocyanides.
CH3NC Methyl isocyanide
CH3CH2NC Ethyl isocyanide
CH3CH2CH2NC Propyl isocyanides
IUPAC name system
In this system, cyanides are named alkyl carbylamines.
CH3NC Methyl carbylamine
CH3CH2NC Ethyl carbylamine
CH3CH2CH2NC Propyl carbylamine
Preparation of isocyanides
Carbylamine reaction
When aromatic and aliphatic primary amines are heated with chloroform and alcoholic KOH, isocyanides or carbylamines are formed, and the reaction is known as a carbylamine reaction. The newly formed material has an unpleasant odor. The synthesis of carbylamine or isocyanide is easily recognized by its disagreeable odor.
In the carbylamine reaction, a primary amine and chloroform are heated with alcoholic potassium hydroxide to produce alkyl isocyanide or carbylamine.
RNH2 + CHCl3 + 3 KOH → RNC + 3 KCl + 3H2O
The compound generated as product RNC is the alkyl isocyanide, and the reaction is known as an isocyanide test or Hoffmann’s carbylamine reaction. Secondary and tertiary amines are not affected by the carbylamine reaction.
From Dichlorocarbene
This is a two-step process. A base reacts with chloroform in the first step to produce dichlorocarbene. Dichlorocarbene converts primary amine into isocyanide in the second step.
Me3CNH2 + CHCl3 + 3 NaOH → Me3CNC + 3 NaCl + 3H2O
From alkyl halide
When ethyl iodide is heated with an ethanolic solution of AgCN, the major product is ethyl isocyanide, while the minor product is ethyl cyanide.
CH3CH2 – Br + AgCN → CH3CH2 – NC + AgBr
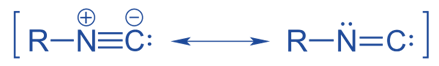
From N -alkyl formamide
N -alkyl formamide reacts with POCl3 in the presence of pyridine to form isocyanides.
Naturally occurring isocyanides
The isocyanide functionality can be found in a variety of organic compounds derived from living organisms. The first was found in an extract from the mold Penicillium notatum Westling in 1957. Later, the antibiotic xanthocillin was developed. Since then, various additional isocyanides have been discovered. Most marine isocyanides are terpenes, but certain terrestrial isocyanides are derived from -amino acids. Many plants and foods contain trace levels of isocyanide, including almonds, apricots, and honey. The gas is also created by the body when proteins are broken down.
Physical properties of isocyanides
- They are colorless, volatile poisonous liquids with a highly offensive smell.
- They are almost completely insoluble in water. This is owing to the absence of a lone pair of electrons in the nitrogen atom in their molecule, which prevents them from forming hydrogen bonds with water. They are, however, soluble in all common organic solvents.
- The boiling points of isocyanides are greater than those of alkyl halides but lower than those of isomeric cyanides.
- They exhibit considerable absorption in their IR spectra between 2165 -2110 cm-1.
- They are also polar, however, they have a lower dipole moment than cyanides.
- They have dipole moments of the order of 3D.
Chemical properties of isocyanides
A cyanide ion is a very powerful electron-drawing group. As a result, it is a good Lewis acid. It generates a complex when it reacts with a Lewis base.
After then, the complex can react with another Lewis base to generate another complex. This can continue indefinitely, generating a chain of complexes. Because of their high electron density, they are extremely reactive. They can react with a wide range of substances. Some reactions of isocyanides are as below:
Hydrolysis
Alkalis do not hydrolyze alkyl isocyanides. They are, however, hydrolyzed by dilute mineral acids to produce primary amines and formic acids.
R -NC + H2O/ H+ → R -NH2 + HCOOH
Oxidation
Alkyl isocyanides on oxidation with ozone produce alkyl isocyanates.
R – N = C + O3 → R – N = C = O + O2
Reduction
When isocyanides are completely reduced, they form secondary amines. The resulting secondary amine has at least one methyl group.
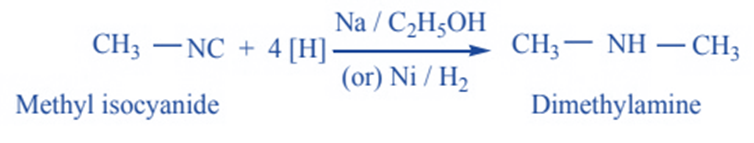
Isomerization
With the developed thermal rearrangement in 1873. The majority of isocyanides isomerize between 200 and 2500 degrees Celsius. The radical inhibitors have the ability to suppress the side reaction (Decyano rxn). Aromatic isocyanides are 10 times faster to isomerize than aliphatic isocyanides. The substituent has little effect on the rate of this reaction. Because the conditions for bond formation and breaking were entirely opposite. The substituent affects the thermal dynamics of this reaction. The σ-accepting or π-donating substituent favored isomerization.

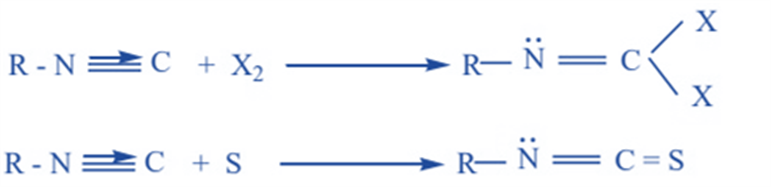
Addition reaction
Alkyl isocyanides react with sulfur and halogen to give corresponding addition products.
Cycloaddition reaction
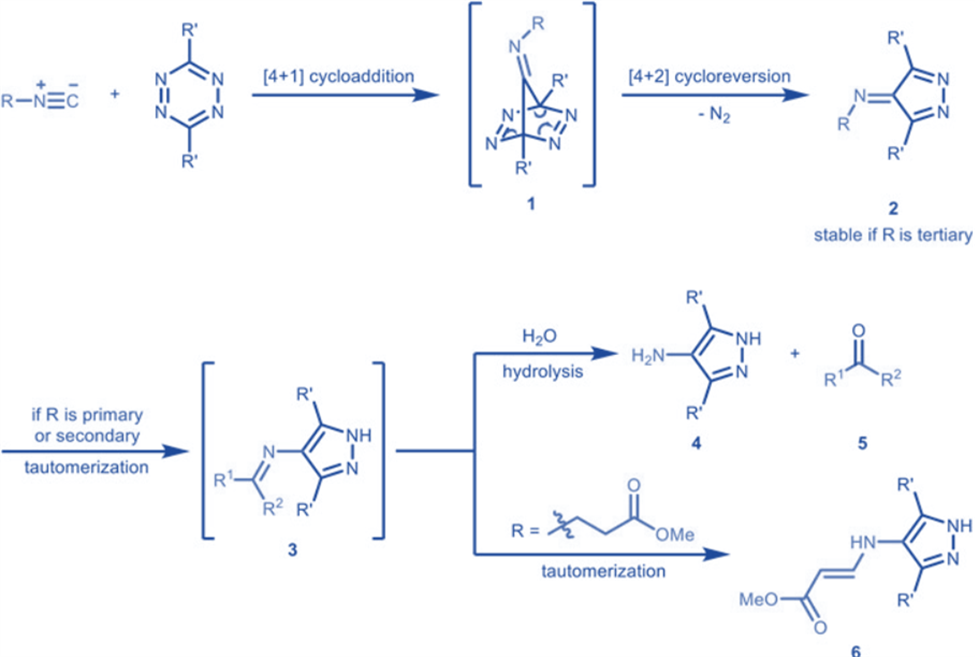
Isocyanides also participate in cycloaddition reactions, such as the 4+1 cycloaddition with tetrazines. This reaction converts isocyanides into carbonyls or produces stable cycloadducts, depending on the degree to which the isocyanide is replaced.
Seitz et al. published the first example of benzyl isocyanide [4 + 1] cycloaddition process using tetrazines to create pharmacologically relevant amino-pyrazoles in 1982. The initial step in this transformation is the production of tetraazanorbornadienimine derivatives 1 by [4 + 1] cycloaddition. This intermediate was unstable and would spontaneously undergo [4 + 2] cycloreversion with the release of N2 to produce imine derivatives 2, which would subsequently tautomerize to aromatic pyrazoles 3 and then hydrolyze to amino-pyrazoles 4 and carbonyls 5. Imines 3 produced from propanoate-derived isocyanides also did not hydrolyze but instead was tautomerized to give the vinylogous urethane derivatives 6.
Reaction with water
They are stable to a stable base (they are commonly produced under clearly basic conditions), but they are sensitive to corrosion. They are corrosive in water. They hydrolyze to formamides in the presence of water. This reaction is used to destroy musty isocyanide mixtures. When exposed to Lewis and Bronsted acids, several isocyanides can polymerize.
RNC + H2O → RN(H)C(O)H
Uses of isocyanides
- They are also employed in the manufacture of polyurethanes. Polyurethanes are a form of material that is utilized in a range of applications such as furniture, insulation, and vehicle parts.
- They are frequently used in organic synthesis due to their high regioselectivity, chemoselectivity, stereoselectivity, and functional group tolerance.
- Isocyanides are commonly used in painting, shipbuilding, firefighting, electrical wire protection, cement, rubbers, and filaments.
- Isocyanates are widely used and found in a variety of industries and activities, including painting, construction, shipbuilding, upholstery manufacturing, and firefighting.
- It’s also used to make insecticides, herbicides, and medicines.
- Isocyanide is a fumigant and an industrial chemical.
- Because of their exceptional reactivity, isocyanides are well-known components of a wide range of natural reactions, particularly the Passerini and Ugi reactions.
Toxicity of isocyanides
Isocyanide is a chemical with a triple carbon-nitrogen bond. Since this type of bond is very reactive, isocyanide is a highly toxic chemical. Isocyanide gas inhalation might result in sudden death due to respiratory paralysis. When a person is exposed to excessive doses of isocyanide, they can develop isocyanide toxicity. This can happen through inhalation, ingestion, or skin absorption. Isocyanide toxicity symptoms include a fast heartbeat, chest pain, shortness of breath, headache, dizziness, and confusion. Isocyanide intoxication can cause unconsciousness or death in severe conditions. People who deal with isocyanide gas are in danger of being exposed to the gas, which can cause eye, nose, and throat irritation as well as difficulty breathing.
References
- https://www.brainkart.com/article/Alkyl-Isocyanides-(Carbylamines)_41403/.
- https://www.chemeurope.com/en/encyclopedia/Isocyanide.html.
- https://infinitylearn.com/surge/chemistry/isocyanide/#:~:text=Isocyanide%20Properties&text=Isocyanides%20are%20a%20group%20of,to%20their%20high%20electron%20density.
- https://www.frontiersin.org/articles/10.3389/fchem.2021.670751/full
- https://www.britannica.com/science/isocyanide.
- https://www.vedantu.com/chemistry/isocyanide.
- https://gbdong.cm.utexas.edu/seminar/old/Isocyanide%20Chemistry_Zack.pdf.
