Ionic solids are a form of crystalline solid. Ionic bonds are formed between positive and negative ions because of their electrostatic attraction to one another. These bonds are what keep the cations and anions that are found in ionic solids together. They are connected in a continuous, three-dimensional system by electrostatic forces. It maximizes electrostatic attraction between opposite charges and minimizes electrostatic repulsion between like charges by positioning the ions.
There are two types of solids: crystalline solids and amorphous solids. In addition, there are four other points next to crystalline solids: covalent network solids, ionic solids, molecular solids, and metallic solids. Anions and cations combine to form ionic solids. Examples of ionic solids include NaCl and CaCO3. Ionic compounds are all generally found in nature as ionic solids.
Interesting Science Videos
Discovery Of Ionic Solids
In 1913, William Henry Bragg, an English chemist, and William Lawrence Bragg, an Australian British physicist, identified the crystal structure of sodium chloride.
It revealed six equally spaced nearest neighbors for every atom, revealing that the constituents were not grouped in molecules or finite aggregates but rather as a network with a long-range crystalline structure. Many more inorganic solids were discovered that have similar structural characteristics.
Evidence that these solids are made up of ions rather than neutral atoms was found in the mid-1920s through X-ray reflection tests, which measure the density of electrons.
Properties of Ionic Solids
Due to the presence of a potent force of attraction between cations and anions, ionic compounds possess the following characteristics:
- Ionic bonds are considered to be the strongest type of chemical bonds.
The ionic bond exhibits high reactivity in appropriate conditions due to its inherent charge separation. - Ionic solids exhibit high melting and boiling points.
- Ionic solids exhibit high electrical conductivity when dissolved in water or melted due to their strong ionic bonding. The presence of ions, which function as charge carriers, accounts for this phenomenon.
- The ionic bonded molecules exhibit certain characteristics as a result of the potent electrostatic attraction between cations and anions.
Formation Of Ionic Solids
Ionic Compounds are formed when metals react with non-metals. Ionic bonds (which form ionic compounds) are basically the electrostatic force of attraction between two oppositely charged atoms. The ionic bond is formed between an anion and a cation. Chemical bonds are formed between a metal and a nonmetal through the electrostatic force of attraction.
Transfer of electrons from one atom to another forms the ionic bond. In this case, one atom can stand for electrons for the inert gas configuration, whereas another atom needs electrons for the inert gas configuration.
For Example: Formation of Ionic Compound NaCl
A neutral Sodium (Na+) atom is likely to get an octet in its outermost shell by giving up one of its valence electrons. As a result, Sodium gives an electron to Chlorine (Cl–) and gets a positive charge. Chlorine will get an electron (a negatively charged particle) from Sodium because it takes less energy for Chlorine to get a stable octet by gaining one electron than by losing seven. So, Sodium is a little bit positive and Chlorine is a little bit negative. The sodium turns into a cation, and the chlorine turns into an anion. So, sodium is made less reactive because it gives up an electron, and chlorine is made more reactive because it gets an electron.
Electrostatic force of pull keeps these two ions together. Thus, in this way sodium chloride formation takes place by the electron transfer.
![Formation of Ionic Compound [Sodium Chloride]](https://scienceinfo.com/wp-content/uploads/2023/05/image-110.png)
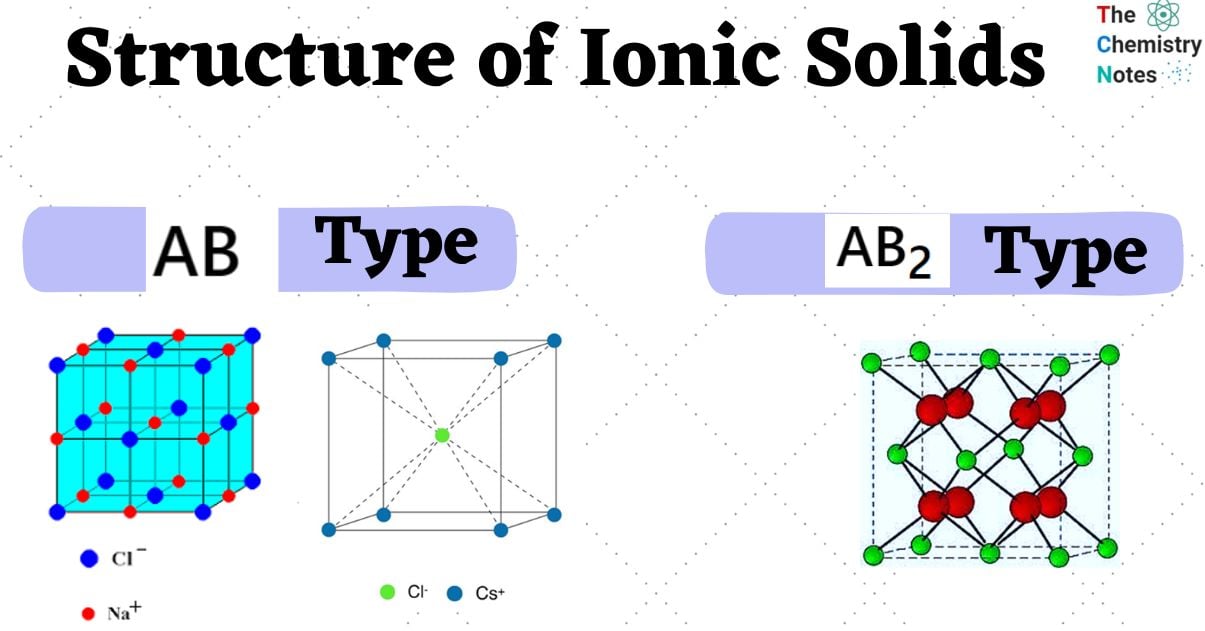
Structure of Ionic Solids
Lattice Structure Of Ionic Solids
A lattice is a three-dimensional configuration of ions or atoms within a crystal. The arrangement of atoms is systematic. This depicts an atom’s basic configuration in general. This “unit,” which can make up the entire structure of the material if repeated a sufficient number of times, is called the lattice structure of the material.
Simple ionic compounds are divided into two types: AB and AB2.
When there are two ions in a binary complex, the anions usually fill the space lattice with hcp or ccp arrangements, while the cations fill the empty spaces between the anions.
- If the crystal structure is made up of anions (B–) and all of the empty spots in an octahedron are filled with cations (A+), then the formula of the ionic solid is AB.
- In the same way, if half of the empty spaces in a tetrahedron are filled with cations, the formula of the solid crystal is A+B–.
- When the anions (B2-) make up the space lattice and the cations (A+) fill all the empty tetrahedral spaces, the solid crystal will have the formula A2B.
Ionic compounds with the type AB
There are three kinds of crystalline shapes for ionic compounds of type AB.
- ZnS [Zinc Blende ]
- NaCl [rock salt]
- CsCl [Cesium chloride]
ZnS [Zinc Blende/zinc sulphide] Type Structure
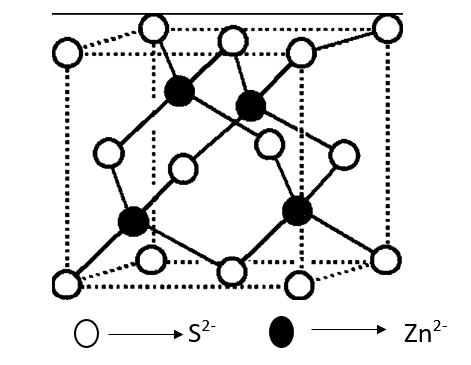
- Crystals of zinc sulfide are made up of the same amount of Zn+2 and S2- ions.
- The sizes of the two ions (Zn+2 = 74 pm and S–2 = 184 pm) led to the radius (r+ / r–) being 0.40, which suggests a tetrahedral shape. rZn+2 / rS–2 = 0.40
- Zinc ions are in a ccp configuration, which means that sulphide ions are at the corners and in the middle of each face of the cube.
- The tetrahedral hole is filled by zinc ions. Only half of the tetrahedral holes are filled with Zn+2, so the formula for zinc sulphide is ZnS. This means that the ratio of zinc to sulphur in the compound is 1:1.
- Since the void is tetrahedral, each zinc ion is surrounded by four sulphide ions, and each sulphide ion is surrounded by four zinc ions. So, zinc sulfide has 4:4 Coordination.
- The ratio of Zn+2/S-2 should be 0.225 for Zn+2 to fit perfectly in the tetrahedral holes made by closely packed S-2 ions. In fact, this ratio is a bit high (0.40) Per unit cell, there are four Zn+2 ions and four S-2 ions, as shown below:
Number of S-2 ions = 8 (at corners) 1/8 + 6 (at face centers) 1/2 = 4
The number of Zn+2 ions in the body is 4 * 1 = 4.
Thus, the number of ZnS units per unit cell is equal to 4. Some more examples of ionic solids having Zinc blende structures are CuC, CuBr, CuI, AgI, beryllium sulphide.
NaCl [Rock Salt] Type Structures
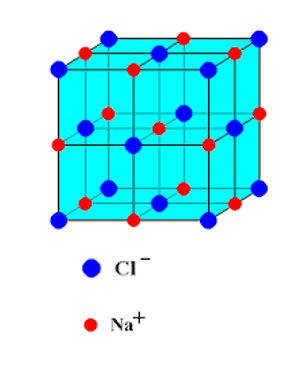
- Ions of Na+ and Cl– make up the structure of sodium chloride.
- The number of Cl– ions is the same as the number of Na+ ions. The Na+ and Cl– ions have radii of 95 pm and 181 pm, respectively. This gives a radius ratio of 0.524. With a radius ratio of 0.524, NaCl seems to have a gap in its octahedral shape.
- In a normal unit cell, the chloride ions are packed together in a way called cubic close packing (ccp). Cl– ions are at the corners and in the middle of each face of the square in this arrangement. This is also known as a face-centered cubic layout (fcc).
- All of the octahedral holes have sodium ions in them. Since the number of Na+ ions is equal to the number of octahedral holes in the ccp structure, every octahedral hole is filled with Na+ ions. So, the chemical formula for sodium chloride is NaCl, or 1:1 stoichiometry.
- Each Cl– ion is surrounded by 6 Na+ ions because there are six octahedral holes around it. In the same way, there are six Cl– ions around each Na+ ion. So, both the Cl– and Na+ ions have a coordination number of six. This is called as 6:6 coordination.
- It should be mentioned that for Na+ ions to fit perfectly into the octahedral holes, the radius ratio rNa+ / rCl– should be equal to 0.414. But the real radius ratio (rNa+ / rCl–= 0.524) is higher than this number. So, to make room for big Na+ ions, the Cl– ions move a little bit apart, meaning they don’t touch each other anymore and form an expanded face-centered lattice.
As shown below, each unit cell of sodium chloride has 4 sodium ions and 4 chloride ions.
At the center of the edges: 12 (at edge centers) × 1/4 + 1 (at body center) × 1= 4
Number of chloride ions = 8 (at corner) 1/8 + 6 (at center of face) × 1/2 = 4
So, there are 4 units of NaCl per unit cell.
Cesium Chloride (CsCl) Type Structures
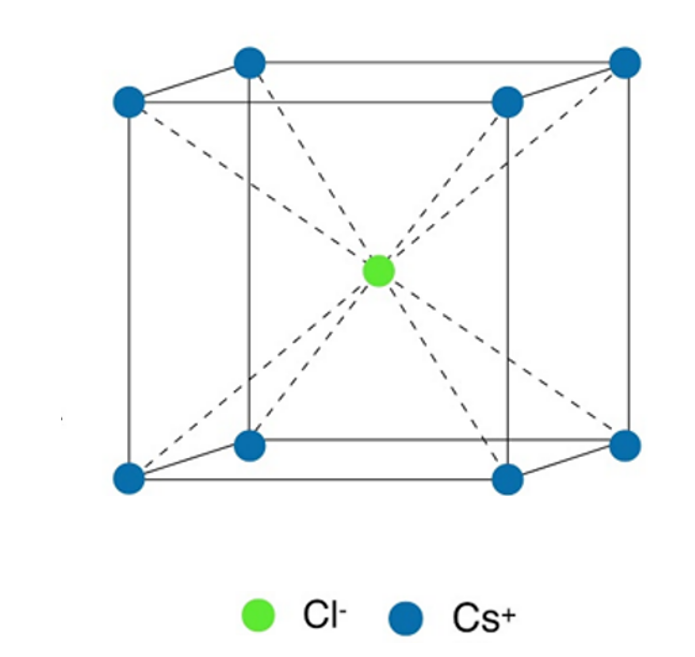
- The crystal of cesium chloride is made up of the same number of ions of cesium (Cs+) and chloride (Cl–).
- The sizes of two ions, Cs+ = 169 pm and Cl– = 181 pm led to a radius ratio of rCs+/ rCl– of 0.93, which suggests a body-centered cubic structure with a cubic hole.
- The chloride ions are arranged in a simple cube, and the cesium ions fill the spaces between the cubes. In other words, the Cl– ions are at the corners of a square, and the Cs+ ions are in the middle, or vice versa.
- Each Cs+ ion is surrounded by eight Cl– ions, and each Cl– ion is surrounded by eight Cs+ ions. So, each ion Coordination number is eight.
- For the Cs+ ions to fit perfectly in the cubic holes, the ratio of rCs+ to rCl– should be 0.732. In reality, though, the ratio is a bit bigger (0.93). So, Cl– ions have to slightly spread out to make room for Cs+ ions.
- Caesium chloride has one Cs+ ion and one Cl– ion in each unit cell, as shown below:
Number of Cl– ions 8 (at corners) divided by 1/8 = 1
No. of Cs+ ions = 1 at the center of the body 1 = 1
So, one unit cell has one unit of CsCl.
This type of molecule is also seen in CsBr, CsI, TlCl, and TlBr
Higher coordination numbers in CsCl (8:8) suggest that the cesium chloride lattice is more stable than the sodium chloride lattice (6:6). It turns out that the lattice of cesium chloride is 1% more stable than the lattice of sodium chloride.
Ionic compounds with the type AB2
Fluorite [CaF2] and rutile [TiO2], are the two most common structures of AB2 type of ionic solids, and many difluorides and dioxides have one of these structures.
Fluorite Structures
![Fluorite Structure [CaF2]](https://scienceinfo.com/wp-content/uploads/2023/05/image-115.png)
- The Ca+2 ions are in a ccp arrangement, which means that they are in all of the cube’s sides and in the middle of each face.
- All of the tetrahedral holes are filled with F– ions.
- Since there are two tetrahedral holes for each Ca+2 ion and F– ions fill all of the tetrahedral holes, there will be two F– ions for each Ca+2 ion, so the stoichiometry of the combination is 1:2.
- Each Ca+2 ion is surrounded by 8 F– ions, and each F– ion is surrounded by 4 Ca+2 ions. The Ca+2 ion has a coordination number of eight, and the F– ion has a coordination number of four. This is called 8:4 Coordination.
- As shown below, each unit cell has 4 calcium ions and 8 fluoride ions.
No. of Ca+2 ions = 8(at corners)×1/8 + 6 (at face centres)´1/2
No. of F– ions = 8 (within the body)×1 = 8
Thus the number of CaF2 units per unit cell is 4.
Similar structure can also be seen in SrF2, BaCl2, BaF2, PbF2, CdF2, HgF2, CuF2, SrCl2.
Factors Affecting Structure of Ionic Solids
There are some of the factors that affect the crystal lattice structure of ionic solids. The two main factors affecting the structure of ionic solids are:
- Charge of the Ions.
- Size of the Ions.
Charge of Ions
The charge of ions affects the structure as similar charges repel each other and opposite charges attract each other. According to the Coulomb’s Law, the stronger the charges of the ions, the more stable is the lattice structure.
Size of Ions
The ionic radius of the ions affects the formation of lattice structure. According to Coulomb’s Law, the smaller the ionic radius, the more stable the lattice and the stronger the electrostatic forces between ions.
Na+ cations are smaller than the Cl– anions The larger anions is located in the outer corner of crystal lattice while the smaller cations fill the space in between.
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Brown, Theodore L, H E. LeMay, Bruce E. Bursten, Catherine J. Murphy, Patrick M. Woodward, and Matthew Stoltzfus. Chemistry: The Central Science. , 2018.
- https://www.flexiprep.com/NIOS-Notes/Senior-Secondary/Chemistry/NIOS-Chemistry-Lesson-6-Solid-State-Part-9.html
- McQuarrie, Donald A.; Rock, Peter A. (1991). General chemistry (3rd ed.). New York: W.H. Freeman and Co. ISBN 978-0-7167-2169-7.
- Pauling, Linus (1960). The nature of the chemical bond and the structure of molecules and crystals: an introduction to modern structural chemistry (3rd ed.). Ithaca, N.Y.: Cornell University Press. ISBN 978-0-8014-0333-0.
- https://www.geeksforgeeks.org/ionic-bonds-and-ionic-compounds/
- https://collegedunia.com/exams/formation-of-ionic-compounds-science-articleid-7253
- Prakash, Satya (1945). Advanced inorganic chemistry. New Delhi: S. Chand & Company Ltd. p. 554. ISBN 978-81-219-0263-2.
- https://www.technologyuk.net/science/matter/ionic-solids.shtml
- https://www.studysmarter.co.uk/explanations/chemistry/ionic-and-molecular-compounds/structure-of-ionic-solids/
- https://www.askiitians.com/iit-jee-solid-state/structure-of-ionic-compounds/

