Ion chromatography (IC) is a separation technique that exhibits several similarities with high-performance liquid chromatography (HPLC). However, IC also possesses distinct characteristics, such as its principle of separation and modes of detection, which allow its independent investigation.
Ion chromatography (IC) is a technique that has been modified to effectively separate ions and polar compounds. The mobile phase consists of an aqueous solution containing ions, while the stationary phase is comprised of an ion-exchange resin. In addition to absorbance or fluorescence-based detection methods, ion chromatography also employs electrochemical techniques that rely on the ionic properties of the substances being separated. The primary advantage of this technique lies in its ability to analyze anions that lack alternative, expedient analytical methods. However, the present-day utilization of ion chromatography extends beyond the examination of basic ions, for which the method initially gained recognition. The operational realm, which can be likened to that of the capillary.
Interesting Science Videos
Ion Chromatography
The determination of organic ions and inorganic cations and anions using ion chromatography (IC) is a common analytical technique. In chromatographic separation, it is regarded as a straightforward approach that is both inexpensive and effective. Sensitivity, specificity, robustness, a low detection limit, and quantification are just several of the positive validation metrics it possesses. Traditional wet chemistry methods, which typically involve more analysis stages, can be easily replaced by IC. In most situations, organic solvents are not needed for the simple extraction of samples, which makes it easier to dispose of the resulting trash. Since its inception, IC has expanded from use solely in university research facilities to include the food, pharmaceutical, and environmental monitoring sectors.
Principle of Ion Chromatography
The interactions of an ion with the resin (the stationary phase) and the eluent (the mobile phase) are the basis of ion chromatography. Both an anion column and a cation column exist in these phases, but they attract opposite types of ions. Each column can only be used to gauge the conductivity of the ion type to which it is most strongly attracted. The ion chromatographer uses the differences in charge and size of the ions to cause the ions to travel through the columns at various rates, allowing for separation. Ions with a lower affinity for the resin will flow through the column more quickly and be eluted ahead of those with a higher affinity, as the eluent is passed through the column.
As the ions leave the column, their electrical conductivity is measured by a special detector. The chromatogram generated by this detector is a function of conductivity vs time. The concentration of each ion in the injected solution determines the height of its respective peak on this graph. Subsequently, these measurements can be employed to ascertain the concentrations of analytes in an unidentified sample.
In order to mitigate potential interference arising from the presence of ions in the mobile phase, it is possible to employ a suppressor mechanism to eliminate the undesired electrolyte prior to conducting the measurement of conductivity. As the eluent solution traverses the suppressor, the ionic species present in the eluent are substituted with a nonionic counterpart. In contrast, in cases where the eluent is adequately diluted or exhibits low conductivity, the employment of a suppressor becomes superfluous.
How Does Ion Chromatography Work?
- Ion chromatography is a type of liquid chromatography that separates ionic species based on how they interact with a resin.
- This type of chromatography is used to determine the concentration of ionic species. Depending on the type of species and its size, ionic species separate in a variety of ways. Sample solutions are forced through a pressured chromatographic column, where the contents of the column are responsible for the absorption of ions.
- When an ion extraction liquid, also known as an eluent, is passed through the column, the ions that have been absorbed start to separate from the column.
- Ionic concentrations in the sample can be calculated based on how long it takes different species to be retained.
Instrumentation of Ion Chromatography
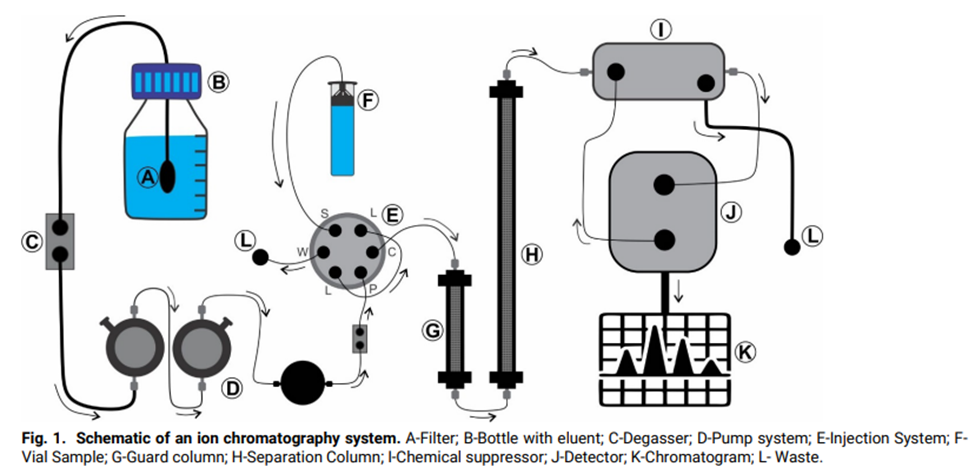
Mobile phase
The eluent is the mobile phase used in chromatography, and it can be created with a fixed or variable concentration using an eluent generator cartridge that contains the required concentrated electrolyte solution. In this eluent, the sample is first dissolved before being transferred to the stationary phase. This facilitates the dissection of a sample solution into its constituent parts.
Different eluent solutions are employed for anion and cation analysis. Anion analysis typically uses a diluted buffer solution of sodium bicarbonate and sodium carbonate as the eluent. The eluent might also be potassium or sodium hydroxide. Dilute acids including sulfuric, acetic, citric, sulfonic, and carboxylic acids are commonly used as eluents in cation analysis.
Degasser
Wear is a means to guarantee the high quality of the eluent because chromatographer performance is substantially influenced by the quality of the eluent. The degasifier is a high-pressure gas removal device that removes electrolysis gases produced while producing eluents, preventing the formation of bubbles caused by the gases outlet in the eluent ratio valves, pump “heads,” and detector cell, which may impair the chromatographic process.
Pump systems
For sensitive detectors like conductivity, UV/VIS, and amperometry, a constant and pulses free-flowing phase is required, and this is what alternative double piston pumps give. As a result, the largest possible residual pulse is dampened using a combination of electrical circuitry and pulse shock absorbers.
Isocratic pumps have fixed capacity and gradients, while others can switch between the two. Although isocratic elution is the method of choice for normal cationic analysis, gradient elution provides the opportunity to separate and analyze a much broader spectrum of ions.
Injector
A valve injector is used to introduce the sample into the system. Six ports in a two-position changing arrangement are characteristic of an injection valve. Injection valves have two positions: one is used to load the sample into the injection circuit, and the other is utilized to move the mobile phase from the guard column to the separation column.
Columns or stationary phase
Chromatographic columns, also known as separation columns, are cylindrical tubes with inlets and outlets at either end, packed with supports. Porous and solid substrate particles having positively charged or negatively charged ionic functional groups on their surface make up the majority of the contents of these columns.
The ion exchange resin, which can be either organic or inorganic, must be stable and insoluble in common solvents. The most common type of ion exchanger is an organic resin. Chromatographic separations are more effective when using those with a silica base. Polystyrene is the most common kind, and it adds divinylbenzene quite simply to create a polymer.
Resins with inorganic qualities have advantageous ion exchange properties. The thermal stability of this resin is improved. Hydrated oxides, acid salts of polyvalent metals, salts of heteropoly acids, insoluble ferrocyanides, and aluminosilicates are the most commonly utilized inorganic ion exchange materials
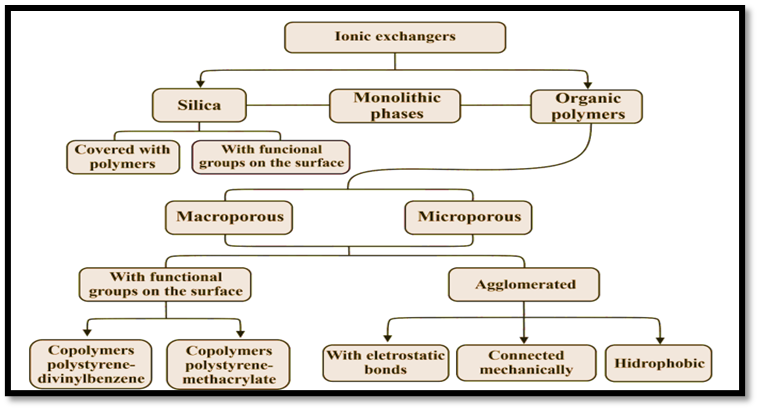
Ion Exchangers
There are three primary categories of ion exchangers across functional categories. Zwitterionic ligands have both charges in a single column, allowing them to bond to both positively and negatively charged molecules in cation exchange chromatography and anion exchange chromatography, respectively.
- Anion exchanger: Resins that can bind anions are known as anion exchangers. The interaction of tertiary amines with styrene-DVB produces strong base resins, while primary and secondary amine groups functionalize weak base resins. Adsorption of strong acids is a possible application, however, the kinetics is slower. Good yields can be expected from amine resins, and they can be regenerated with NaOH, NH4OH, or NaCO3.
- Cation exchanger: The organic polymeric matrix of cationic ion exchangers can be either robust or brittle. Sulfonation, whose functional group is sulfonic acid, is used to create strong acid resins. These resins have a wide pH tolerance but high re-generant needs. This is the method of choice for virtually all water testing. Carboxylic resins with exchange capability are known as weak character exchangers and are used to neutralize a wider range of organic ions.
- Zwitterionic exchanger: Zwitterionic ion exchangers refer to the stationary phases employed in the multi-selective retention mechanism. The exchangers possess both positive and negative charges, resulting in an overall neutral charge. Zwitterionic stationary phases are characterized by the accumulation of equimolar quantities of oppositely charged functional groups, which are either situated in close proximity to the surface or distributed throughout the volume of the stationary phase. The zwitterionic phase can be conceptualized as a phase that comprises an equal proportion of highly acidic and highly basic functional groups.
Suppressors
Prior to detection, a suppressor cell is present following the process of sample separation. The primary purpose of the electrochemical suppressor system within the detection unit is to diminish the background signal generated by the mobile phases, thereby enhancing the detectability of sample ions.
Consequently, the elevated electrical conductivity of the electrolytes present in the eluent is mitigated by implementing a discerning exchange membrane. This intervention effectively diminishes the baseline and facilitates the detection of substances at low concentrations.
Detector
The analyte is transported to the detector of the apparatus by passing through the suppressor. There exist multiple categories of detectors, including conductivity, amperometric, potentiometric, spectrophotometric, and fluorescence detectors.
UV-VIS detection is widely utilized in high-performance liquid chromatography (HPLC), however, its usage in ion chromatography (IC) is restricted. The utilization of the fluorescence detector in ion chromatography is infrequent due to the limited number of ions that exhibit fluorescence properties. Amperometric detection is applicable for samples exhibiting pK values exceeding 7. The utilization of refractive index detection in integrated circuits (IC) is infrequently observed.
Mass spectrometry (MS) is widely recognized as one of the most efficient methods for detection. However, the coupling of the IC with this particular detector is not commonly observed, primarily due to its relatively high cost in comparison to the other detectors mentioned.
Conductivity cells are widely employed in the field of ion chromatography (IC) to determine the presence of anions and cations. This is achieved by measuring the electrical conductance of the analyte, which produces a signal that is indicative of the physical or chemical properties of the sample. The resulting data is then processed by a computer system to generate a chromatogram.
Procedure for Ion Exchange Chromatography
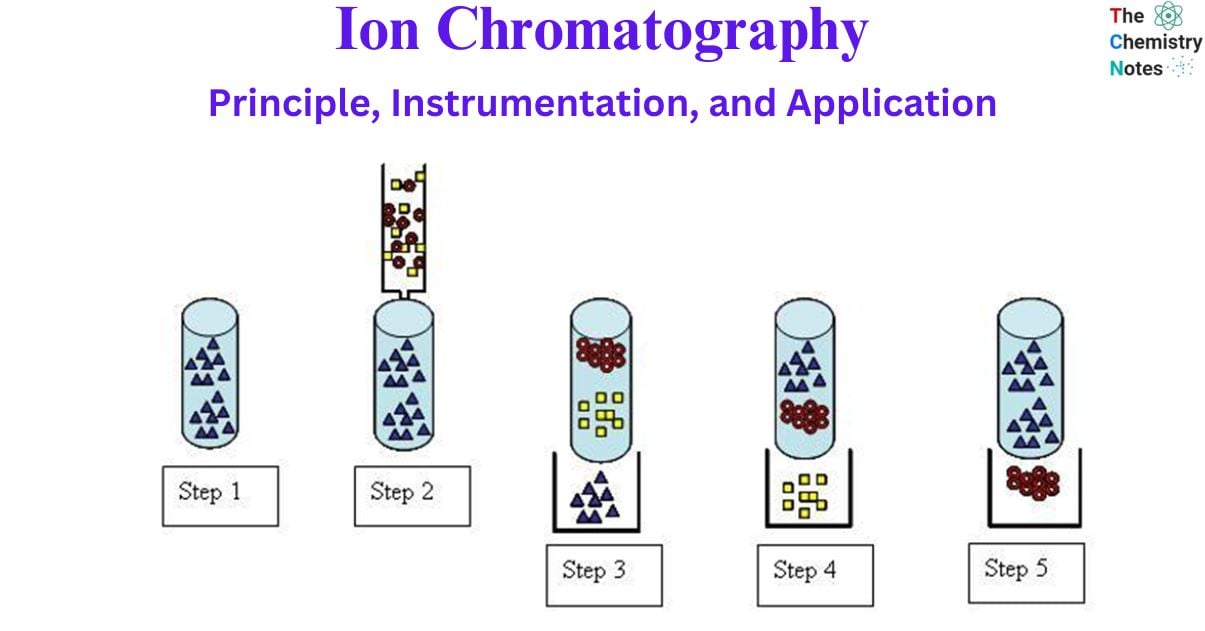
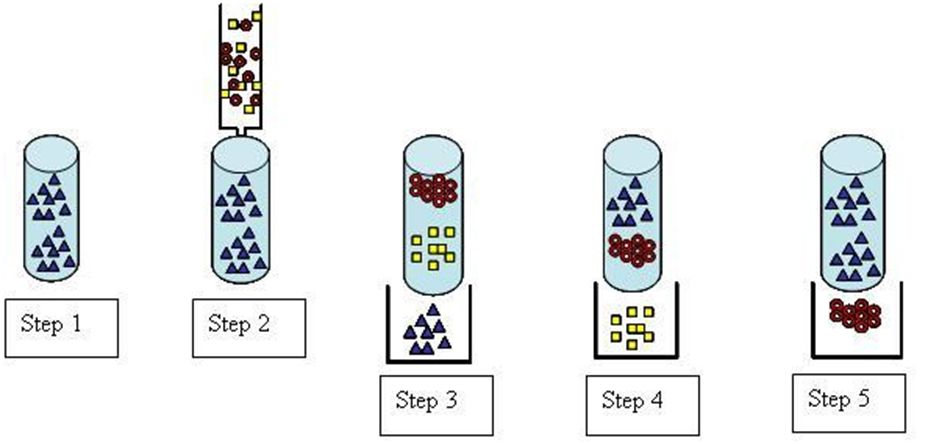
Below is a diagram and explanation of the five stages that make up the fundamental process of ion exchange chromatography: eluent loading, sample injection, separation of the sample, elution of analyte A, and elution of analyte B. The process of elution involves the transport of the target component along the column. This occurs due to the constant pumping of the eluent through the column, which is the solvent used in chromatography. The chemical reactions below are for an anion exchange process.
- To displace any other anions bound to the resin, run the eluent anion through the column. During this loading phase, the eluent anion is used to completely saturate the resin surface. Remember, if you want consistent chromatographic outcomes, you need to make sure all the column’s ionic sites are filled with eluent ions before you run the analysis.
- Small amounts of A- and B- containing samples (often less than 100 L) are injected into the column. This sample could contain a wide variety of ions; however, just two are used here for clarity.
- Ion exchange allows A– and B– to flow through the column after the sample has been injected by continuously adding eluent anion to the mobile phase.
- A– migrates in a band across the column as the eluent is applied and finally elutes out of the column.
- B– is finally eluted from the column as a result of the eluent.
Sample preparation
The implementation of a sample preparation procedure is essential prior to conducting IC analysis to safeguard the integrity of the separation columns and ensure the attainment of dependable and consistent outcomes. An inadequately prepared sample has the potential to induce several undesirable effects on the chromatographic system. These effects include increased column back pressure, diminished retention times, decreased peak efficiency, compromised resolution, impaired reproducibility, irregular baseline, and contamination of detector cells. Consequently, the overall performance of the instrument may be significantly altered.
- The selection of the sample preparation technique should aim to achieve optimal recovery of the analyte while simultaneously minimizing the presence of interfering substances.
- The techniques employed vary in complexity, encompassing straightforward procedures such as sample dilution, filtration, or pH modification, as well as more intricate protocols that entail multiple steps.
- These complex methods include analyte extraction from solid materials, analyte pre-concentration, and sample purification steps aimed at eliminating interferences.
In the field of analytical chemistry, it is not possible to directly analyze samples that contain highly acidic or basic substances. Therefore, it is necessary to subject these samples to acid digestion and subsequently partially neutralize them before they can be injected for analysis. Nevertheless, the inclusion of a neutralizing base hinders the identification of the introduced cation and results in an elevation of the salt burden, thereby potentially disrupting chromatographic separations.
One potential solution involves the utilization of ion exchangers, either in microcolumn or membrane-based setups. These ion exchangers, when in their acidic or basic states, have the ability to neutralize alkaline or basic solutions, respectively. This neutralization process can be achieved without the need for additional reagents or dilution.
For liquid samples
- Before running a sample through an ion chromatograph, it is best practice to filter it to remove silt and other particulate matter and to reduce the likelihood of microbial modification.
- Water samples must be collected in a sterile syringe or bottle and filtered through a 0.45um (or smaller) filter before analysis.
- Additionally, the collection vial needs to be washed out three times with filtrate before it is filled to the brim with sample filtrate.
- Until they can be analyzed, samples require cold storage.
- There is no maximum sample size requirement and the minimum necessary for analysis is about 5mL.
For solid samples
- Ions on the surface of a solid sample can be removed using water or acid (cations). To prepare liquid samples for examination, they must be filtered and kept refrigerated. There are no upper limits on the size of a solid sample, although the minimum size is about 2–3 centimeters.
Advantages of Ion Chromatography
- One of the primary benefits associated with ion chromatography is its capability to simultaneously analyze multiple ions, owing to the distinct elution rates exhibited by each ion.
- In addition, the duration required for sample analysis in the context of ion chromatography (IC) is generally estimated to be approximately ten minutes, thereby establishing IC as a highly efficient analytical technique.
- In contrast to instrumental approaches, such as ion-selective electrodes, which can be susceptible to matrix interference, standard addition methods do not require implementation.
Some other advantages of using this chromatography involve:
- Fast
- High selectivity
- Good accuracy and precision
- High selectivity
- High speed
- High separation efficiency
- Good tolerance to sample matrices and
- Low cost of consumables
Application of Ion Chromatography
- It is employed for the analysis of water chemistry and the identification of chemical contaminants, particularly in drinking water.
- This analytical technique is employed for the quantification of sugar and salt concentrations in various food products.
- This technique is employed for the purpose of selectively isolating specific proteins.
Conclusion
The use of ion exchange chromatography has contributed to the method’s development and is responsible for a number of its benefits. In recent years, it has seen an increase in use, which has led to an increase in its popularity for use in the analysis of water, pharmaceuticals, food, and the monitoring of the environment. The method possesses desirable degrees of precision and accuracy, a broad range of applications, a multitude of detecting modes, excellent selectivity, speed, separation efficiency, and a low cost for consumables. However, like every approach, it has some limits dependent on the use of the method, which can be lessened through the hyphenation of other techniques. However, these limitations are not as severe as the limitations of other techniques.
References
- Varão Moura, A.; da Silva, J. D. S.; Gubert, P. Ion Chromatography: Principles and Instrumentation. Orbital: Electron. J. Chem. 2022, 14, 110-115
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Analytical_Sciences_Digital_Library/Courseware/Separation_Science/07_Specialty_Topics/Ion-exchange_Chromatography/03_Basic_Principles_of_Ion_Chromatography
- https://serc.carleton.edu/microbelife/research_methods/biogeochemical/ic.html
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Analytical_Sciences_Digital_Library/Contextual_Modules/Effects_of_Acid_Rain_on_Atlantic_Salmon_Populations/05_Ion_Chromatography
- https://research.cbc.osu.edu/reel/research-modules/environmental-chemistry/instrumentation/instrument-calibration/ion-chromatography-theory/
- https://pradeepresearch.org/ion-chromatography/
