Enthalpy (H), is a property commonly used to describe the thermodynamics of chemical and physical processes. It is the total of a thermodynamic system’s internal energy as well as the volume times pressure ratio. It is a property or state function that is similar to energy; it has dimensions similar to energy (and is therefore measured in units of joules or ergs), and its value is solely dependent on the temperature, pressure, and composition of the system, rather than on its history.
Interesting Science Videos
What is Enthalpy?
Enthalpy is a measure of a system’s overall heat content and is equal to the system’s internal energy plus the sum of its volume and pressure. In a thermodynamic system, energy is quantified as enthalpy.
At constant pressure, the change in enthalpy equals the heat absorbed or released during the process.
The word “enthalpy” is derived from the Greek words for “warming“.
Enthalpy Change
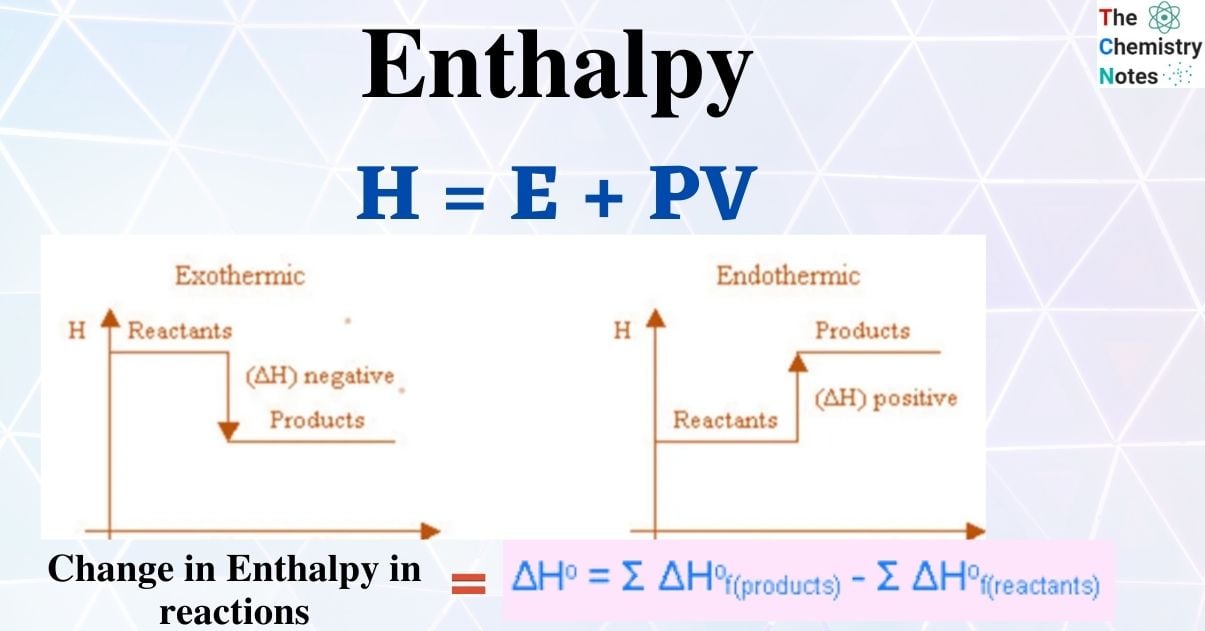
The enthalpy of a substance is its total chemical energy (or heat content). Chemical energy changes during chemical reactions, which causes the enthalpy to change. It is denoted by ∆H.
∆H may be either positive or negative.
Hess’s law is employed to compute enthalpy changes:
The enthalpy change of a process is equal to the sum of the enthalpy changes of each individual step if it can be expressed as the sum of several steps.
We can calculate the change of an overall reaction by adding the ∆H value of the individual reactions that make up a chain reaction.
The following abbreviated version of Hess’s law can be used to calculate if we are aware of the standard enthalpies of formation, or ΔH, of the reactants and products:

Where Σ ΔH stands for the total change in enthalpies.
According to Hess’s Law, whether you convert reactants A into products B in one step, two steps, or however many steps, the overall enthalpy change will always be the same.
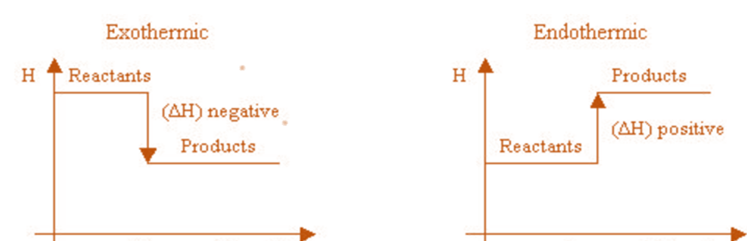
When a reaction releases energy, it is exothermic, and ΔH is a negative number. But when a reaction absorbs energy, it is said to be endothermic, so the ΔH value is positive.
Standard Enthalpy of Reaction
We already know that the enthalpy of any reaction depends on the environmental physical conditions, such as temperature, pressure, etc. Any reaction’s standard enthalpy can be calculated when all of the reactants and products are present in their standard forms, which is necessary to specify the enthalpy of any reaction. In light of this, the standard enthalpy of reaction is the change in enthalpy that takes place in a system when a substance undergoes a chemical reaction under normal circumstances.
The symbol for a reaction’s standard enthalpy is ΔH°.
Conventionally, any substance’s pure form at a pressure of 1 bar is considered to be its standard state at a given temperature. As an illustration, liquid ethanol is said to be in its standard state in its pure form when it is 298 K and 1 bar of pressure.
Calculation of Enthalpy Change
The enthalpy of the products and reactants are used to calculate ∆H in the simplest manner possible. If you are aware of these values, you can calculate the overall change using the following formula:
∆H = H products − H reactants
Illustration:
One reaction that can be calculated in this manner is the addition of a sodium ion to a chloride ion to produce sodium chloride. H values for sodium ions are 239.7 kJ/mol for ionic sodium and 167.4 kJ/mol for chloride ions. The value for sodium chloride (table salt) is 411 kJ/mol. Adding these values results in:
= −411 kJ/mol – (−239.7 kJ/mol −167.4 kJ/mol)
or, −411 kJ/mol – (−407.1 kJ/mol)
or, −411 kJ/mol + 407.1 kJ/mol
= −3.9 kJ/mol
Thus, each mole of salt produced releases almost 4 kJ of energy.
Mathematical Definition
Introducing the idea of enthalpy (H), which is referred to as the mathematical product of the volume (V), pressure (P), and internal energy (U) of a system (V)
Hsys = Esys + PV
Hence, for a simpler understanding, we will sum up the relationship between the system’s internal energy and enthalpy as follows:
H = E + PV
During a chemical reaction, ∆H of the system equals the change in its internal energy plus the change in the product of the pressure times the volume of the system. (∆H) is a measure of how much energy is transferred during processes that occur under constant pressure, which is a common circumstance for many chemical and physical changes.
∆H = ∆E + ∆(PV)
As a result of the reaction being conducted at constant pressure, the change in enthalpy that takes place during the reaction is equal to the change in the system’s internal energy plus the product of the constant pressure times the change in the system’s volume.
∆H = ∆E + P ∆V (at constant pressure)
The first law of thermodynamics can be substituted into this equation to produce the following outcome.
∆H = (qp + w) + P∆V
The P∆V terms in the equation cancel when it is assumed that the reaction’s only work is expansion-related.
∆H = P∆V + (qp – P∆V)
Thus, the change in the system’s enthalpy equals the heat produced or absorbed by a chemical reaction occurring at constant pressure.
∆H = qp (at constant pressure)
This is the most practical choice for determining heat changes for chemical reactions typically experiments are conducted under typical atmospheric conditions, at constant external pressure, with ∆H = qp .
Relationship between the Enthalpy and the Change in Internal Energy
- When a reaction is run at constant volume, the amount of heat released or absorbed equals the change in the system’s internal energy.
∆Esys = qv
- The change in the system’s enthalpy equals the amount of heat that is released or absorbed when a reaction is carried out under a constant pressure.
∆Hsys = qp
- A chemical reaction causes H to change, and this change is equal to the internal energy of the system plus the product of the gas’s volume and pressure.
∆Hsys = ∆Esys +∆ (PV)
For reactions involving only liquids and solids, there is little to no change in the volume , which results in a small difference between ∆E and ∆H.
However, if the number of moles of gas changes during the reaction, the difference can be quite significant involving gases.
Signs in Enthalpy (ΔH)
An exothermic reaction is indicated by a negative enthalpy change value (H < 0), while an endothermic reaction is indicated by a positive value (H > 0). When a chemical equation’s direction is switched, its H’s arithmetic sign is altered (a process that is endothermic in one direction is exothermic in the opposite direction).
To describe the changes in both matter and energy, chemists use a thermochemical equation. The enthalpy change of a reaction is indicated in a thermochemical equation by a H value that appears after the reaction’s equation. This “H” value represents the amount of heat generated by the chemical equation’s reaction involving the specified number of moles of reactants and products.
The physical states of the reactants and products must be demonstrated because they affect the ∆H of a reaction. For instance, 286 kJ of heat are released when 1 mole of hydrogen gas and 12 moles of oxygen gas combine to form 1 mole of liquid water at the same temperature and pressure. A maximum of 242 kJ of heat are released if gaseous water forms.
H2 (g) + 12O2 (g )⟶ H2O(g) ΔH = −242kJ
Specific Enthalpy
The formula for a uniform system’s specific enthalpy is
h = H / m
where m denotes the system’s mass. The specific enthalpy unit in the SI is called joule per kilogram. h = u + pv, where u is the specific internal energy, p is the pressure, and v is the specific volume, and 1/ρ, where the density is can be used to express it in other precise quantities.
Difference between Enthalpy and Specific Enthalpy
- The internal energy and pressure-volume work done make up enthalpy, whereas specific enthalpy is enthalpy per unit mass.
- Because H is a broad property, the size of the matter affects how much it is worth and specific enthalpy is an intensive property, meaning that its value depends only on the type of the system or matter and not on its size or mass.
- In SI units, H is measured in Joules or Kilojoules whereas the units of specific enthalpy are joules/kg or kilojoules/kg.
Importance
- It is significant because it tells us how much heat there is in a system (energy). Heat is crucial because it allows us to produce useful work. In terms of a chemical reaction, an enthalpy shift reveals how much enthalpy was gained or lost.
- It is employed to figure out how much heat a chemical reaction produces.
- In calorimetry, heat flow is measured using the change in enthalpies.
- It is measured to assess a Joule-Thomson expansion or throttling process.
- To determine the minimum power required for a compressor.
- When the state of matter changes, ∆H changes as well.
- Also, it has numerous other uses in thermal engineering.
References
- Atkins, Peter and de Paula, Julio; Physical Chemistry for the Life Sciences, United States, 2006.Katherine Hurley
- Petrucci, et al. General Chemistry Principles & Modern Applications. 9th ed. Upper Saddle River, NJ: Pearson Prentice Hall, 2007
- Treptow, Richard S. “How Thermodynamic Data and Equilibrium Constants Changed When the Standard-State Pressure Became 1 Bar ” J. Chem. Educ. 1999 76 212.
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Thermodynamics/Energies_and_ Potentials
