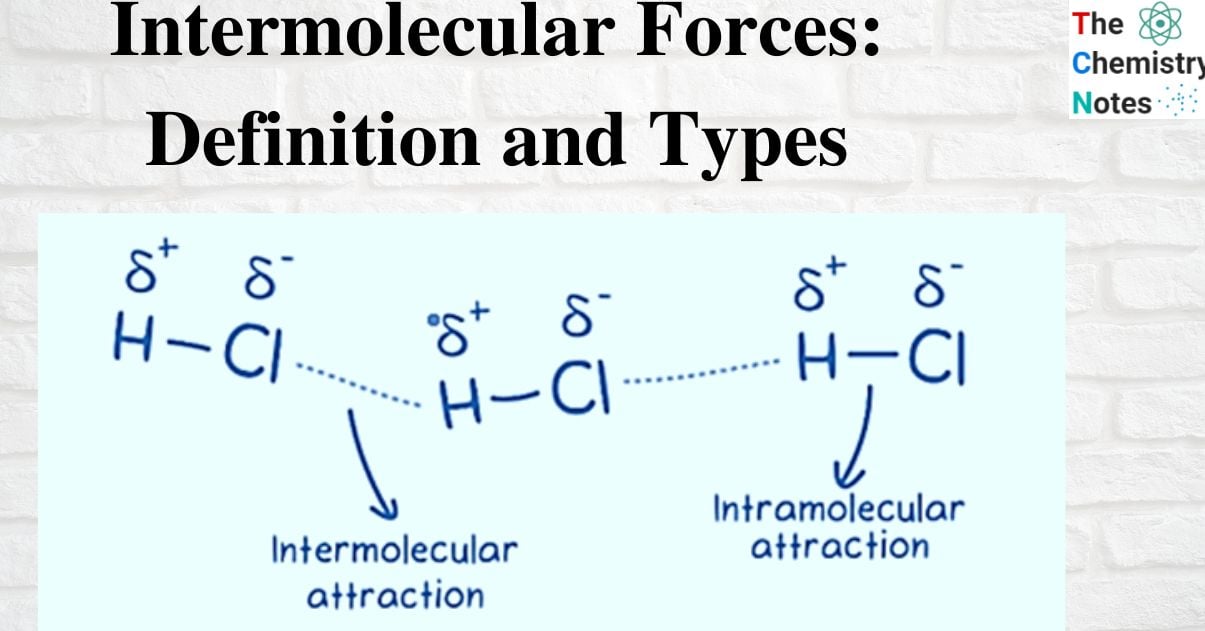
Intermolecular forces (IMF) are the attractive and repulsive forces that exist between the molecules of a substance. IMFs, or intermolecular forces, are mechanical forces that exist between molecules. Contrarily, intramolecular forces are the forces that exist between the atoms of a single molecule. The interactions between molecules (or atoms) are largely responsible for many physical characteristics as well as how molecules are arranged within a substance’s structure.
Interesting Science Videos
What is Intermolecular Force?
Intramolecular forces are the bonds that hold atoms together in a molecule, such as covalent, ionic, and metallic bonding.
Intermolecular forces, on the other hand, refer to the attraction that happens when molecules are close to one another.
Depending on how much attraction exists between molecules, intermolecular forces can be classified as either weak or strong. A greater distance between two or more molecules is the result of weak intermolecular forces. particularly in contrast to strong intermolecular forces, in which molecules exhibit a strong attraction to one another.
Intermolecular forces are especially important in many organic molecules because they contribute to the function, characteristics, and properties of various substances. Although an individual intermolecular force might be viewed as being weak in isolation, the combined strength of all of these intermolecular forces adds to the overall strength of the substance.
Types of Intermolecular Forces
Intermolecular forces can be divided into five categories:
- Dipole-Dipole Interactions
- Ion-Dipole Interactions
- Ion Induced Dipole Interactions
- Dipole Induced Dipole Interaction
- Dispersion Forces or London Forces
Dipole-Dipole Interactions
Dipole-dipole forces exist between polar molecules—those with a permanent dipole moment due to unequal electron sharing. Due to variations in the electronegativities of the atoms involved in a covalent bond, polar molecules have permanent dipoles. One molecule’s partially positive portion will gravitate toward another molecule’s partially negative portion.
- As a result of this unequal distribution, the molecule has a partial positive charge (+δ) on one side and a partial negative charge (δ-) on the other.
- Materials with dipole-dipole attractions tend to have higher melting and boiling points compared to nonpolar molecules, which only have LDF.
- forces between dipoles have strengths per mole ranging from 5 kJ to 20 kJ.
- They are significantly weaker than ionic or covalent bonds and only have a noticeable impact when the molecules are close to one another.
- Since dipoles are permanent, dipole forces are typically stronger than dispersion forces.
- Individual molecules’ polarities tend to align by opposites, drawing molecules together and favoring a condensed phase.
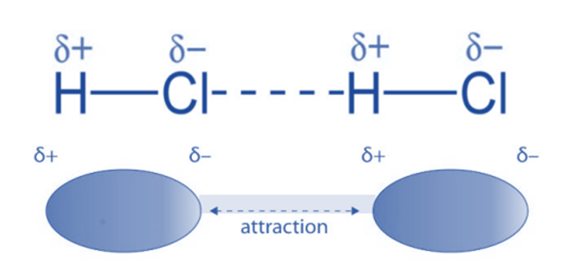
For Example: In HCl molecules, instance, dipole-dipole interactions take place. Chlorine acquires a partial negative charge because it is relatively more electronegative than hydrogen (whereas hydrogen acquires a partial positive charge). The HCl molecules then engage in a dipole-dipole interaction.
Ion-Dipole Interactions
Ion-dipole interaction occurs when an ion encounters a polar molecule.
The part of the molecule that attracts and repels in this situation depends on the ion’s charge. Exactly like the dipole-dipole interaction, the ion-dipole interaction is also very similar. Bonds are formed between polar molecules and ions, and this is the only difference that can be seen.
The following factors determine the strength of the ion-dipole interaction:
- The magnitude of the dipole moment
- Size of the polar molecule
- Dimensions and charge of an ion
- The negative portion of a molecule would attract a cation or positive ion and the positive portion would repel it. The positive and negative parts of a molecule would attract and repel an anion, or negative ion.
- Ion-Dipole Forces are attractive forces between oppositely charged ions and a molecule containing a dipole.
- The magnitude of the dipole molecule (how strong the dipole is) or the strength of the ion’s charge determines the strength of the ion-dipole forces.
Example of Ion-Dipole Interaction
The interaction between a Na+ ion and water (H2O) is an illustration of an ion-dipole interaction because the oxygen atom and sodium ion are attracted to one another, whereas the sodium and hydrogen are repelled by one another.
Consider putting sodium chloride (NaCl) into the water to dissolve it. The sodium-chlorine bond will break up entirely. Water counteracts the sodium ion’s positive charge. Assume that six water molecules are required to balance the charge of sodium. You might picture the sodium ion as being in the middle. The six water molecules will be evenly distributed all around it. Each water molecule has an oxygen atom that is partially negative and points in the direction of sodium. The six water molecules and the sodium interact electrostatically in a balanced manner.
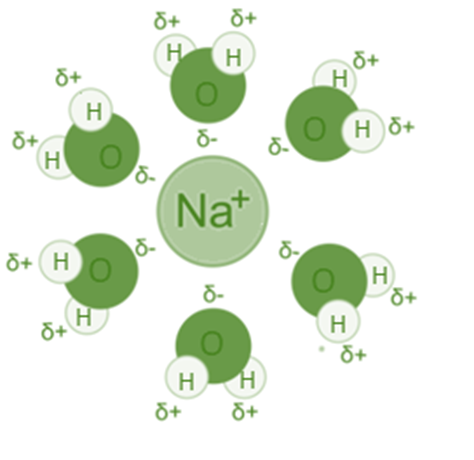
- The water molecules arrange their oppositely charged dipole to be attracted to the fully charged ion when an ionic compound like NaCl dissolves in water, creating a very strong attractive force known as the ion-dipole force.
- The partially negatively charged (-) water molecule is drawn to the positively charged sodium ion (Na+), which is fully charged.
- The water molecules partial positive (+) charge is drawn to the fully charged negative chloride ion (Cl– ).
The ion’s charge is significantly higher than the dipole’s charge. The ionic charge thus acts as a deciding factor. On the periodic table, it rises as we pass from one period to the next. Compared to sodium, magnesium, for instance, has a stronger ion-dipole interaction. The size of the molecule affects how far apart the ion and dipole are from one another. A polar molecule with a few atoms will be able to approach the ion with ease. It will be more challenging for a polar molecule with hundreds of atoms to approach the ion.
Induced – Dipole Forces
Induced dipole forces result when an ion or a dipole induces a dipole in an atom or a molecule with no dipole. These forces are weak.
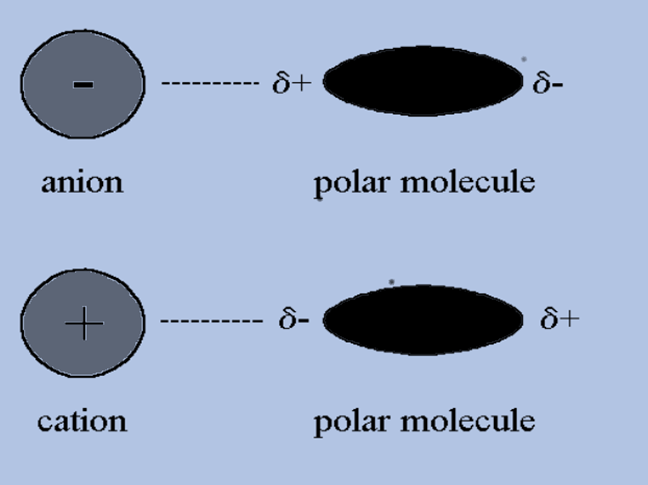
Ion-induced Dipole Forces
When an ion approaches, it can cause a dipole to form in an atom or a non-polar molecule by disrupting the way the electrons are arranged in the non-polar species. This is known as an ion-induced dipole attraction, and it is a weak form of attraction.
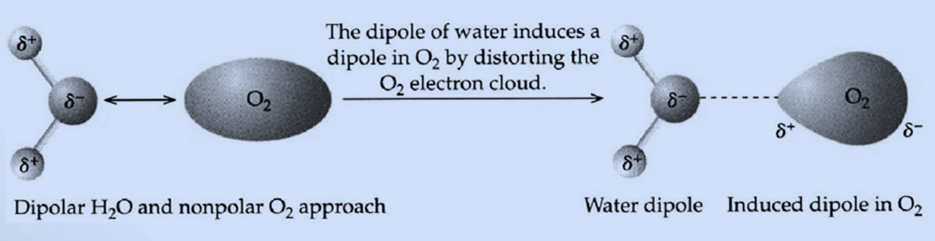
Dipole-Induced Dipole Forces
When a polar molecule disturbs the arrangement of electrons in a nonpolar species, it induces a dipole in an atom or a nonpolar molecule, producing a weak attraction known as a dipole-induced dipole attraction.
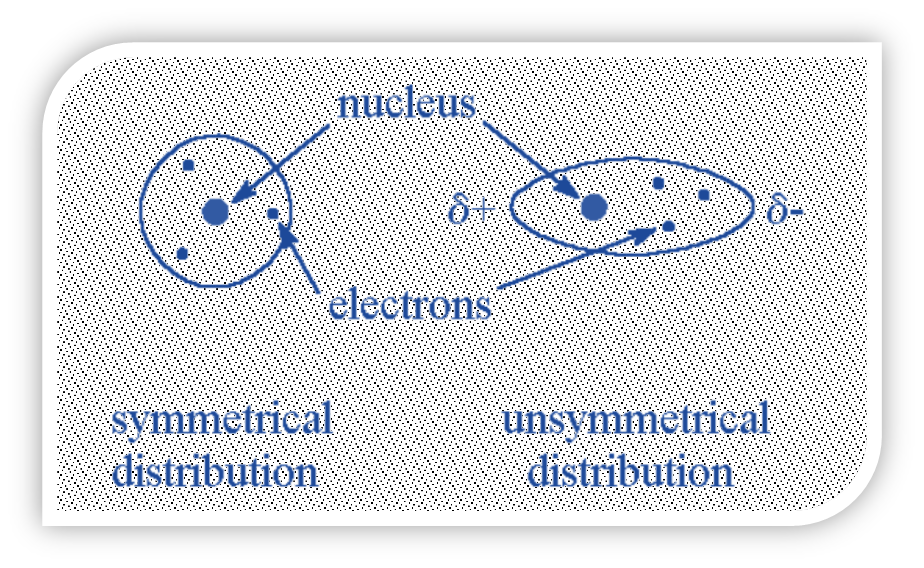
Dispersion Forces or London Forces
A weak intermolecular force between two atoms or molecules that are close to one another is known as the London dispersion force. When two atoms or molecules approach one another, their electron clouds repel one another, creating a quantum force.
Fritz London gave the first explanation of how noble gas atoms could be attracted to one another in 1930, giving the force its name. The second-order perturbation theory served as the foundation for his explanation. Dispersion forces, instantaneous dipole forces, and induced dipole forces are other names for London forces (LDF). Van der Waals forces are a colloquial term for London dispersion forces.
- When an atom or molecule’s electrons are distributed asymmetrically around the nucleus, an instantaneous (temporary) dipole can form due to the electron’s constant motion.
- London dispersion forces are typically stronger between molecules that can be easily polarized and weaker between molecules that can’t.
- The attractive forces known as London forces cause nonpolar substances to condense into liquids and freeze into solids when the temperature is sufficiently lowered.
- As the temperature is lowered, the London dispersion force, which is the weakest of the van der Waals forces, causes nonpolar atoms or molecules to condense into liquids or solids. The dispersion force is typically dominant, despite being the weakest of the three vans der Waals forces (orientation, induction, and dispersion). Small, easily polarized molecules like water molecules are an exception
Dispersion and dipole-dipole attraction forces are collectively referred to as van der Waal forces. It is necessary to first understand the concepts of dipole, polarization, and polarizability in order to understand the nature of these forces.
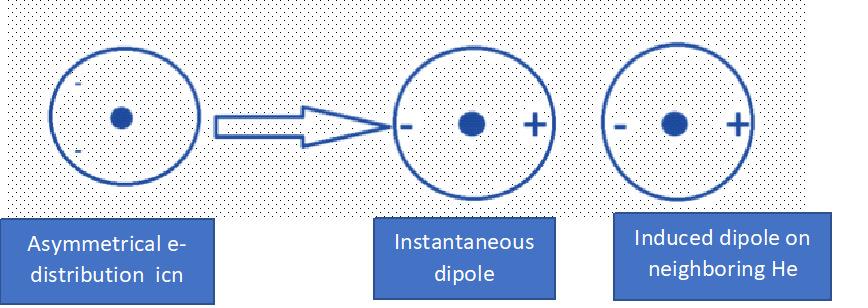
For Example: Dispersion Interaction with a dipole on one He atom induces a dipole on another nearby He atom.
Order of strength of Intermolecular forces
The strength of different types of intermolecular forces in the order of weakest to strongest are listed below:
Dispersion force or London forces (Weakest)
Dipole-dipole force
Hydrogen bond
Ion-dipole force. (Strongest)
References
- Atkins, P., 1989. General Chemistry. 1st ed. New York: Scientific American Books, pp.258-264.
- (BBC – Higher Bitesize Chemistry – Bonding, structures and properties : Revision, Page2, 2018)
- Sharwood, J., 2007. Nelson chemistry. 1st ed. South Melbourne, Vic.: Thomson Nelson, pp.111-116.
- https://byjus.com/chemistry/different-types-of-intermolecular-forces/
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-The_Central_Science(Brown_et_al.)/11%3A_Liquids_and_Intermolecular_Forces/11.02%3A_Intermolecular_Forces
- https://opentextbc.ca/introductorychemistry/chapter/intermolecular-forces/
- https://www.khanacademy.org/science/class-11-chemistry-india/xfbb6cb8fc2bd00c8:in-in-states-of-matter/xfbb6cb8fc2bd00c8:in-in-intermolecular-forces/a/intramolecular-and-intermolecular-forces
