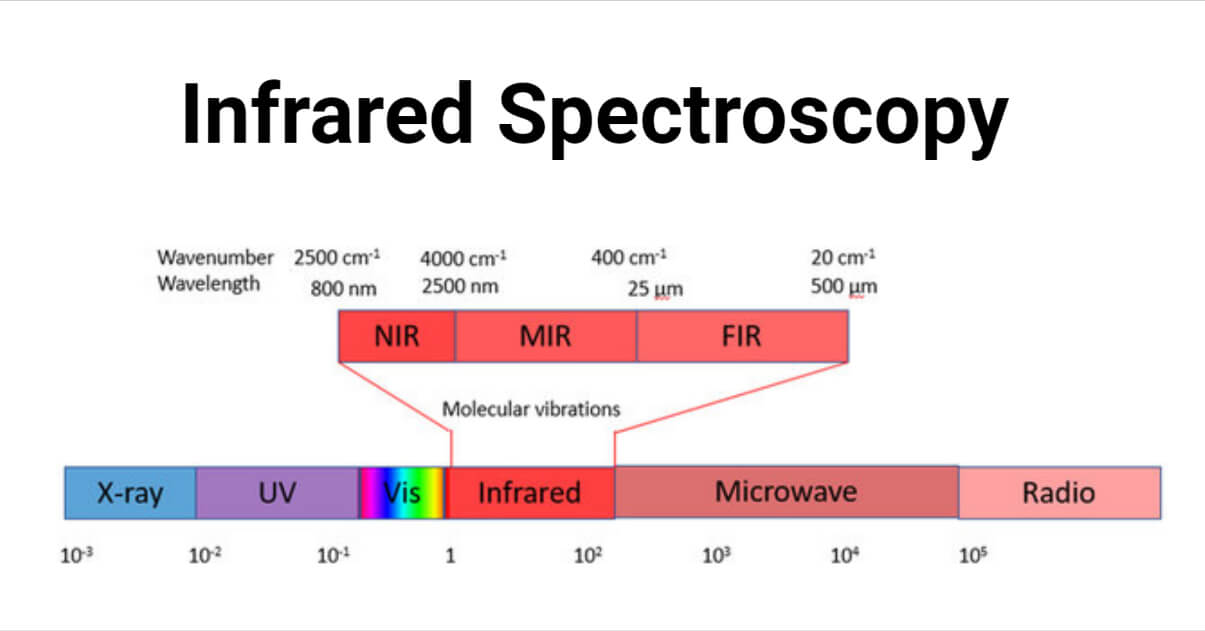
Infrared spectroscopy is the measurement of the interaction of IR radiation with compounds. IR region involves the range between region 400-4000 cm-1. IR radiation causes the excitation of molecules from lower to higher vibrational levels. These vibrational levels are associated with closely spaced rotational levels. So IR spectra are also known as vibrational rotational spectra. The bonds accompanied by a change in dipole moment are IR active.
Requirements for Infrared Spectroscopy
- Compounds should have a dipole moment.
- Two compounds will never show similar IR spectra if they are not enantiomers.
Principle of Infrared Spectroscopy
When IR radiation is passed through the IR active compounds, they will get excited and show specific vibrational rotational spectra, which are characteristic to the functional group of compounds.
The frequency and wavelength of absorption relative to the mass of the atom, force constant of bonds and gemetry of atoms. The band position in IR spectra are represented as wavenumber i.e. ⊽ .
⊽= 1/λ(wavelength)
Transmittance (T) represents the bond intensities.
T= I/Io
where, I= intensity of transmitted light
Io= intensity of incident light
Types of Vibrations
There are generally two types of vibrations. They are:
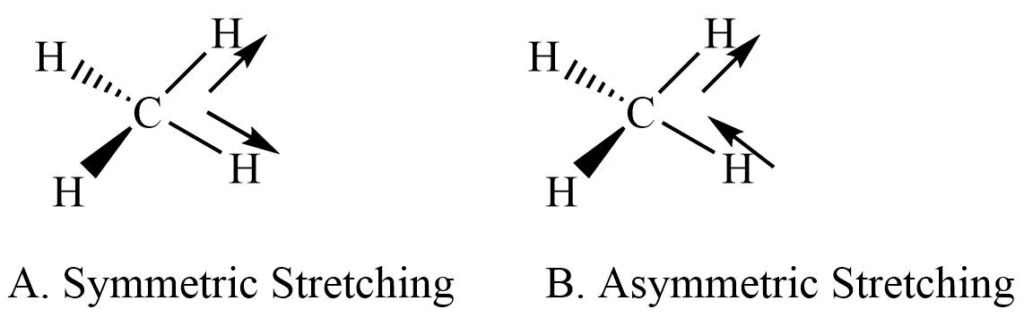
Stretching vibrations
It involves the rhythmical movement along the bond axis. The interatomic distance is increasing or decreasing. It is further subdivided into symmetric and asymmetric stretching.
a. Symmetric stretching: Movement 0f atom with respect to the particular atom in same direction.
b. Asymmetric stretching: One atom approaches the central atom while the other departs from it.
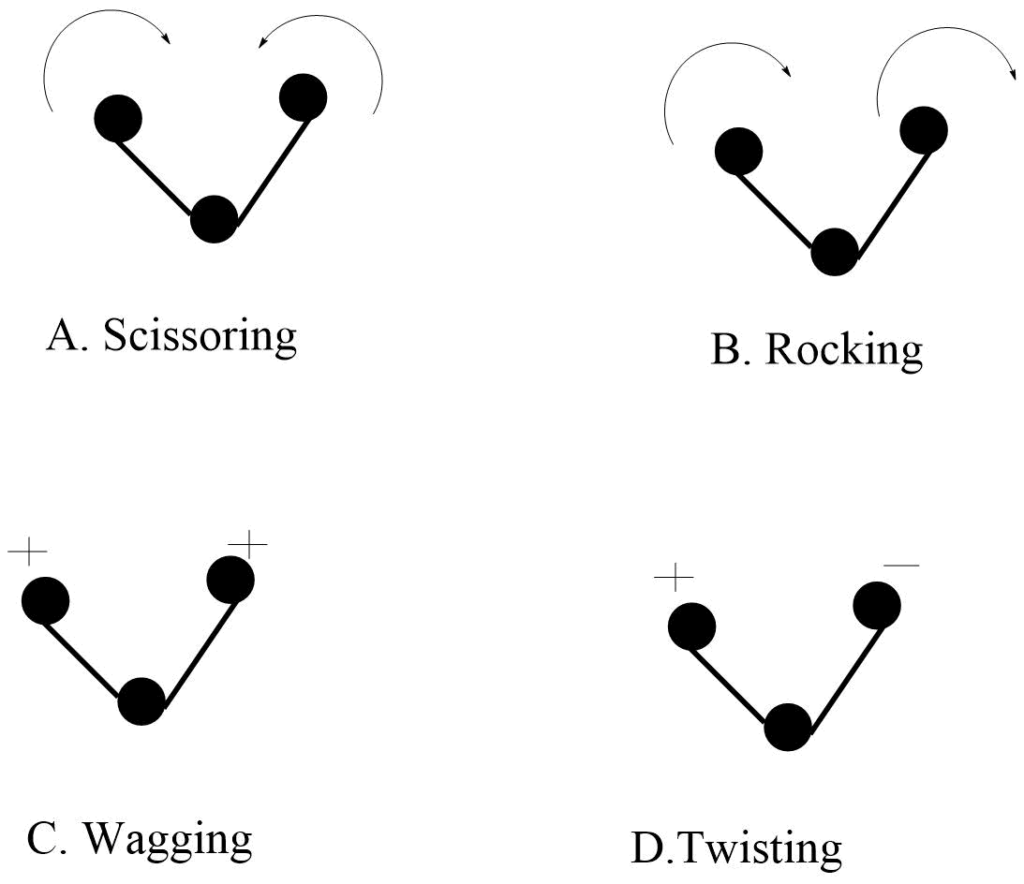
Bending Vibration
In this type of vibration, the position of the atom changes with respect to the original bond axis. There are four types of bending vibrations.
a. Scissoring vibration: Two atoms approaches each other.
b. Rocking vibration: The movement of atoms takes place in the same direction.
c. wagging vibration: Two atoms move up and down the plane with respect to the central atom.
d. Twisting vibrations: In this type of vibration, one of the atoms moves up the plane while the other moves down to the plane with respect to the central atom.
The value of the stretching vibrational frequency can be calculated by the application of Hook”s law. Which is represented as:
⊽= 1/2πC √k/μ
μ= Reduced mass i.e., (m1.m2/m1+m2)
k= Force constant
Different regions of IR spectra
- Functional group region: It involves the region between 1500-4000 cm-1. The characteristic stretching frequency of different functional groups of compounds occurs in this region. The absence of absorption on the functional group’s specific assigned region indicates the absence of such a functional group.
- Fingerprint region: The region below 1500 cm-1 is called the fingerprint region. It is rich in many absorption bands and soulders caused by bending vibrations. Two compounds with the same functional group have a similar IR absorption band above 1500 cm-1, but their IR spectra differ in the fingerprint region. As a result, the fingerprint region is also used to distinguish between different compounds.
Factors affecting IR absorption
- Inductive effect: The alkyl group (+I) effect results in bond weakening, and thus the force constant decreases. which causes a decrease in absorption wavenumber.
Formaldehyde (HCHO) = 1750 cm-1
Acetaldhyde (CH3CHO) = 1745 cm-1
Acetone (CH3COCH3) = 1715 cm-1
The effect of the electronegative group (-I) causes an increase in bond order, which raises the force constant, leading to an increase in the absorption wavenumber.
Acetone (CH3COCH3) = 1715 cm-1
Chloroacetone (CH3COCH2Cl) = 1725 cm-1
Dichloroacetone (CH3COCHCl2) = 1740 cm-1
- Field effect: In ortho-substituted compounds, a lone pair of electrons on two atmos interact with each other in space, changing the vibrational frequencies of both groups.
- Hydrogen bonding: Hydrogen bonding causes a remarkable downward frequency shift. The stronger the hydrogen bonding, the greater the absorption shits towards a lower wavenumber than the normal value. in dilute solution aliphatic alcohols, the sharp band appears at 3650 cm-1 due to the presence of the free -OH group, while broadband is noticed at 3350 cm-1 in concentrated solution due to the presence of hydrogen-bonded -OH group.
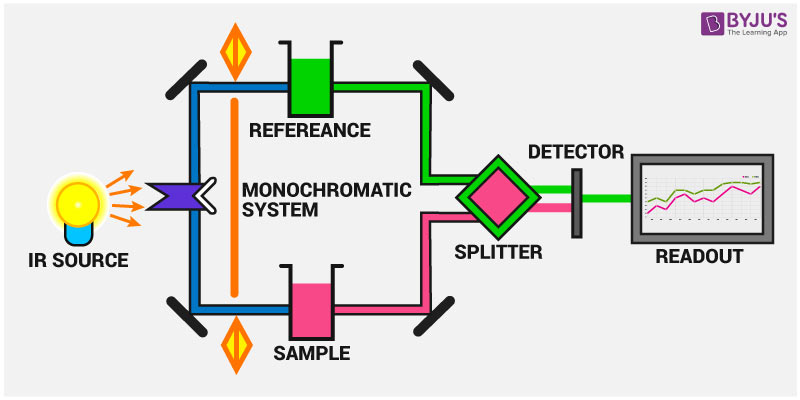
Infrared Spectroscopy Instrumentation
IR spectroscopy measures the vibration of atoms caused by the interaction of IR radiation with the compounds. Based on this, the functional groups present in the compounds can be determined.
The main parts of the IR spectroscopy include:
1. IR radiation source
2. Sampling of substances
3. Monochromator
4. Detectors
5. recorders
Instrumentation of IR spectroscopy is shown in the figure below:
IR radiation sources
IR spectroscopy requires a radiation source capable of emitting IR radiation that is consistent and intense enough to be detected.
Nernst glower
It is a hollow rod made up of oxides of zirconium, yttrium, and erbium. It is capable of producing intense infrared radiation with a broad IR range.
Glober source
It consists of a rod of the sintered silicon carbide. In glober source heating is required to produce IR radiation. The IR radiation produced by glober source is comparatively less intense.
Sampling technique
Various techniques can be employed for placing the sample in the path of IR radiation depending upon whether the sample is a gas, liquid, or solid. The sample of the same substances shows a shift in the frequency of absorption as we pass from the solid, liquid to a gaseous state.
Solid
Solid samples are examined as the alkali halide mixture. The sample is ground with alkali metal like sodium chloride and is made disc after pressing under elevated temperature and high pressure. The disc of pure sodium chloride is placed in the path of a reference beam. The IR spectrum of the solid can be determined as a mull and paste. Nujol and hexachlorobutadiene are the common mulling agents. The sample under investigation should be completely dry as the water absorbs strongly at about 9710 cm-1 and near to 1630 cm-1.
Liquid
The sampling technique for the liquid sample involves the squeezing liquid sample in between two plates of sodium chloride. The pair of sodium chloride plates are placed on the path sample beam.
Gases
The gaseous sample is introduced into the gas cell made up of sodium chloride and placed in the path of the sample beam.
Solutions
The sample solutions are most convenient to determine the spectrum in the solution. The excellent should have poor absorption of own at IR region. The most commonly used solvents are chloroform, carbon tetrachloride, and carbon disulfide.
Monochromater
Different types of monochromators like a prism, grating, and filters are used in IR spectrometers. The prisms are made up of alkyl halides like sodium chloride, and potassium bromide while the filters are made up of lithium fluoride. Gratings are usually made up of potassium bromide.
Detectors
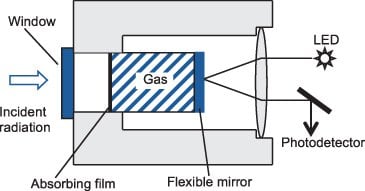
Gloay detector
It is a photoconductivity detector having excellent sensitivity at room temperature. Due to low thermal conductivity xenon gas is normally used in gloay cell detectors.
Bolometer
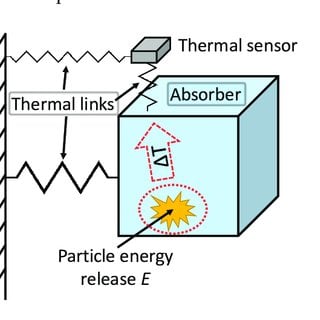
It is a thin metallic conductor which measures the radiation by monitoring the temperature rise of a blackened metal strip. It consists of the temperature-sensitive resistant element, which resistance changes with the temperature. In this detector, an increase in temperature causes an increase in the resistance so current change occurs, which is detected.
Decay_Experiments_Review_and_Prospects/figures?lo=1
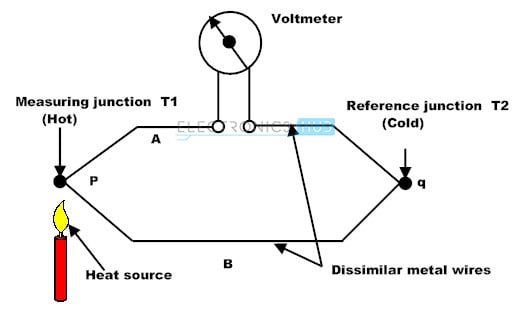
Thermocouple detector
It is made up of two different wires that are linked together. When IR radiation strikes one end, the temperature of that end rises, resulting in the temperature difference between the two ends. The temperature difference between the two ends causes a potential difference, which causes current flow.
Thermistor detectors
They’re made of cobalt, manganese, and nickel sintered oxide. The resistance in these detectors decreases with increasing temperature, resulting in a change in current. Then the current is recorded.
Recorder
A recorder is used to record the IR spectrum.
For more details on the instrumentation of IR spectroscopy watch the vedio given below:
Applications of IR spectroscopy
- Identification of organic compounds: The identity of organic compounds can be established from the fingerprint region. If the fingerprint region of an unknown compound exactly matches the fingerprint region of the known compounds, the identity of the compound is confirmed.
- Analysis of functional groups: Different functional groups produce different IR absorption bands. As a result, IR spectra can be used to identify functional groups.
- Conformational analysis: This technique is quite useful in determining the relative stability of various conformations of cyclic compounds.
- Geometrical isomerism: It is also used for the distinction between cis and trans isomers.
- This technique is also used to distinguish between intramolecular and intermolecular types of hydrogen bonding and to study chemical reactions.
References
- Silverstein, R. M., Webster, F. X., & Kiemle, D. J. (2005). Spectrometric identification of organic compounds. Hoboken, NJ: John Wiley & Sons.
- https://byjus.com/chemistry/infrared-spectroscopy/
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/infrared/infrared.htm
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Spectroscopy/Vibrational_Spectroscopy/Infrared_Spectroscopy
- https://www.savemyexams.co.uk/as/chemistry/cie/22/revision-notes/4-analysis/4-1-analytical-techniques/4-1-1-infra-red-spectroscopy/
- https://microbenotes.com/infrared-ir-spectroscopy/
- https://www.pharmatutor.org/pharma-analysis/analytical-aspects-of-infra-red-spectroscopy-ir/instrumentation-of-ir-spectrophotometry
- https://www.circuits-diy.com/temperature-sensor-circuit-using-thermistor/
- https://www.britannica.com/technology/thermal-detector
- https://www.britannica.com/technology/simple-machine
- https://www.spiedigitallibrary.org/eBooks/FG/Field-Guide-to-Terahertz-Sources-Detectors-and-Optics/50/Golay-Cells/10.1117/3.952851.ch50?SSO=1

