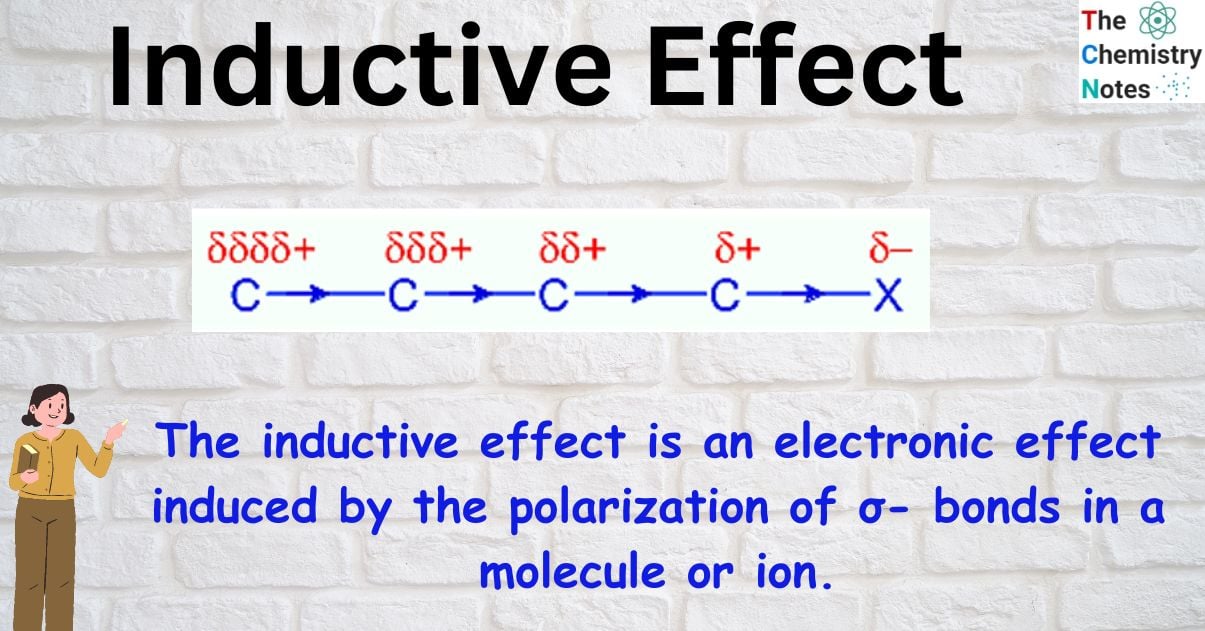
The inductive effect is an electronic effect induced by the polarization of σ- bonds in a molecule or ion. In most cases, this is because of a disparity in electronegativity between the atoms forming the two ends of the bond. When the electronegativity of two atoms is different, the sigma bond between them becomes polarized as the bond pair moves to the more electronegative atom. The electronegative atom receives a partial negative charge and the electropositive atom receives a partial positive charge in this process. As a result, the sigma bonds in the molecule create a persistent dipole that transmits the induced polarity. Inductive effect is a term used to describe this phenomenon.
What is Inductive Effect?
When atoms with varying degrees of electronegativity combine by a covalent bond, the electron density is more concentrated on the more electronegative atoms. This shift in electron density is called the inductive effect.
Because of the uneven distribution of the bonding electrons in a molecule, a permanent dipole is created. Unlike the electromeric effect, which can only occur in pi bonds, this effect can occur in sigma bonds.
It is represented by an arrow pointing in the direction of the more electronegative atom that possesses partial negative charge.

Important Characteristics of the Inductive Effect
- Electronegativity differences between two atoms in a sigma (σ) bond are responsible for the inductive effect.
- A persistent dipole may be formed in the molecule as a result of the inductive action, which persists for a considerable amount of time.
- This effect is weak and occasionally dominated by other electron processes like resonance, hyperconjugation, and so on.
- The inductive effect is caused by the difference in electronegativity between the two atoms in a sigma bond and has a chemical and physical impact on the compounds.
- The strength of the inductive effect diminishes as one moves away from the groups that are producing it.
- It is transferred via sigma bonds. Pi bonds have no role in this.
Types of Inductive Effect
The inductive effect can be either electron-withdrawing or electron-releasing, depending on the nature of the atom or group that induces it. The strength of the inductive effect is determined by comparing it to that of hydrogen.
- Negative inductive effect or -I effect
- Positive inductive effect or +I effect
Negative inductive effect or -I effect
An electron-withdrawing property of a group or atom is known as its negative inductive effect (-I). The symbol for this is -I. When an electronegative atom, such as a halogen, is introduced into a chain of atoms (typically carbon atoms), the unequal distribution of electrons results in the transmission of a positive charge through the chain.
It causes a permanent dipole to form in the molecule when the electronegative atom is negatively charged.The following groupings are listed in decreasing order of their -I effect:
The order of reactivity is as follows:
NH3+ > NO2 > CN > SO3H > CHO > CO > COOH > COCl > CONH2 > F > Cl > Br > I > OH > OR > NH2 > C6H5 > H.
Positive inductive effect or +I effect
The second type of inductive effect is designated by the symbol +I and describes the properties of electron-releasing groups or atoms. The positive inductive effect, or +I effect, occurs when a chemical species that tend to release or donate electrons, such as an alkyl group, is added to a carbon chain, causing the charge to be transmitted through the chain. Here are some examples of groups in order of decreasing +I effect.
C(CH3)3 > CH(CH2)2 > CH2CH3 > CH3 > H
Uses/ Applications of Inductive Effect
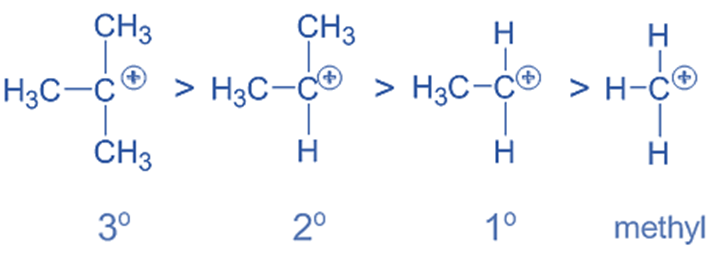
- Evaluating the stability of alkyl carbocation
Carbocations become more stable when +I groups, such as alkyl groups are located close to positively charged carbon. To neutralize the carbon’s positive charge, the +I groups donate negative charge density. Carbocation becomes more stable as a result. Whereas, the -I groups make the carbocations less stable by increasing the positive charge while lowering the electron density.
Note that any factor that increases the charge (positive or negative) on an ion causes destabilization, while any factor that decreases the charge causes stabilization.
The order of stability of a few carbocations containing alkyl groups is as follows:
- Carbanion Stability
In terms of carbanion stability, the +I groups are less stable than the -I groups. For instance, the alkyl groups (+I) will increase carbanion instabilities by transferring electron density to the negatively charged carbon. As a result, carbanions are stable in the following order:
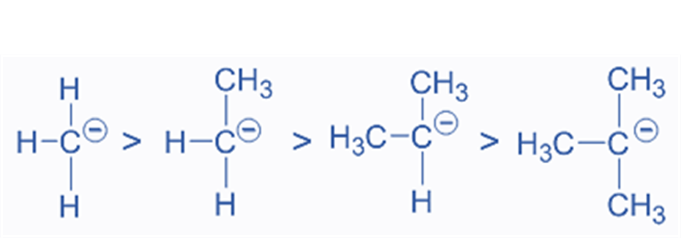
Among the possible carbanions, the tertiary carbanion is the most unstable, while the methyl carbanion is the most stable.
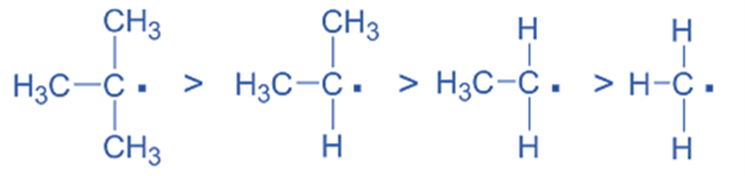
- Stability of Free Radicals
Similarly, an increase in the amount of alkyl groups enhances the stability of free radicals. Therefore, the relative stability of various free radicals is:
- To evaluate the acidic strength of aliphatic carboxylic acids
The strength of a carboxylic acid depends on how much its ionization constant has changed: the more ionized the acid is, the stronger it is. A higher concentration of acid results in a lower pKa value. The alkyl group in acids has an inductive effect that releases electrons, increasing the electron density of oxygen and preventing the oxygen-hydrogen bond from being broken. The pKa of formic acid is 3.74, making it more acidic than acetic acid, which has a pKa of 4.76. Monochloroacetic acid (pKa=2.82) induces ionization and is more effective than formic acid due to chlorine electron-withdrawing action.
- The basic strength of amines
Amines are more basic when they contain electron-donating groups like alkyl groups, and less basic when they contain electron-withdrawing groups like aryl groups. Since alkyl amines are more stable than aryl amines, the former are more effective Lewis bases than ammonia.
Therefore, alkyl and aryl amines are weaker than ammonia in order from least to most basic.
CH3NH2 > NH3 > C6H5NH2
- Reactivity of carbonyl compounds
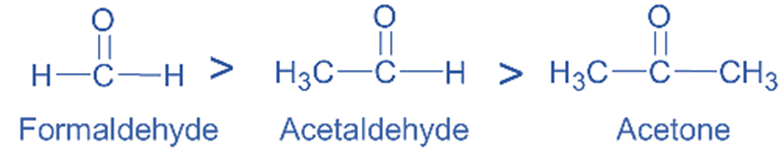
The +I groups increase the electron density at the carbonyl carbon, which increases the reactivity of carbonyl compounds. As a result, they are less reactive with nucleophiles. When compared to acetaldehyde and acetone, formaldehyde is more reactive in nucleophilic addition processes.
References
- https://unacademy.com/content/neet-ug/study-material/chemistry/application-of-inductive-effect/
- Richard Daley (2005). Organic Chemistry, ISBN 978-1-304-67486-9.
- Salvatella, Luis (2017). “The alkyl group is a –I +R substituent” doi:10.1016/j.eq.2017.06.004.
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Stock, Leon M. (1972). “The origin of the inductive effect”. Journal of Chemical Education. doi:10.1021/ed049p400. ISSN 0021-9584.
- https://collegedunia.com/exams/inductive-effect-types-nature-effects-and-differences-chemistry-articleid-4323#Ty
- https://byjus.com/jee/inductive-effect/#Applications-of-Inductive-Effect
