Hydrophobic interaction chromatography is one of the most popular methods for isolating and purifying proteins in their natural state. It has also shown to be quite useful for isolating protein complexes as well as studying protein folding and unfolding. Hydrophobic interaction chromatography is a successful separation technique for removing proteins with minimal loss of biological activity as it uses less denaturing conditions and matrices.
Interesting Science Videos
What is Hydrophobic interaction chromatography (HIC)?
Hydrophobic interaction chromatography (HIC) is a column chromatographic separation method that is commonly used to purify and isolate macromolecules such as proteins and polynucleotides. Proteins are divided by HIC based on variations in the hydrophobicity of their surface. In this process, hydrophobic stationary phase ligands linked to the resin surface interact reversibly with protein molecules.
The running buffer has a significant impact on how hydrophobic proteins interact with a HIC resin. The interaction is improved by a high salt content and goes reverse when the salt content is lower. The protein’s hydrophobic regions and the bound phase are encouraged to bind by using a salt solution as a powerful eluent. The protein is allowed to elute by progressively lowering the salt concentration during the run (normally to 0%), and in HIC, elution is typically done in the order of least hydrophobic to most hydrophobic molecules.
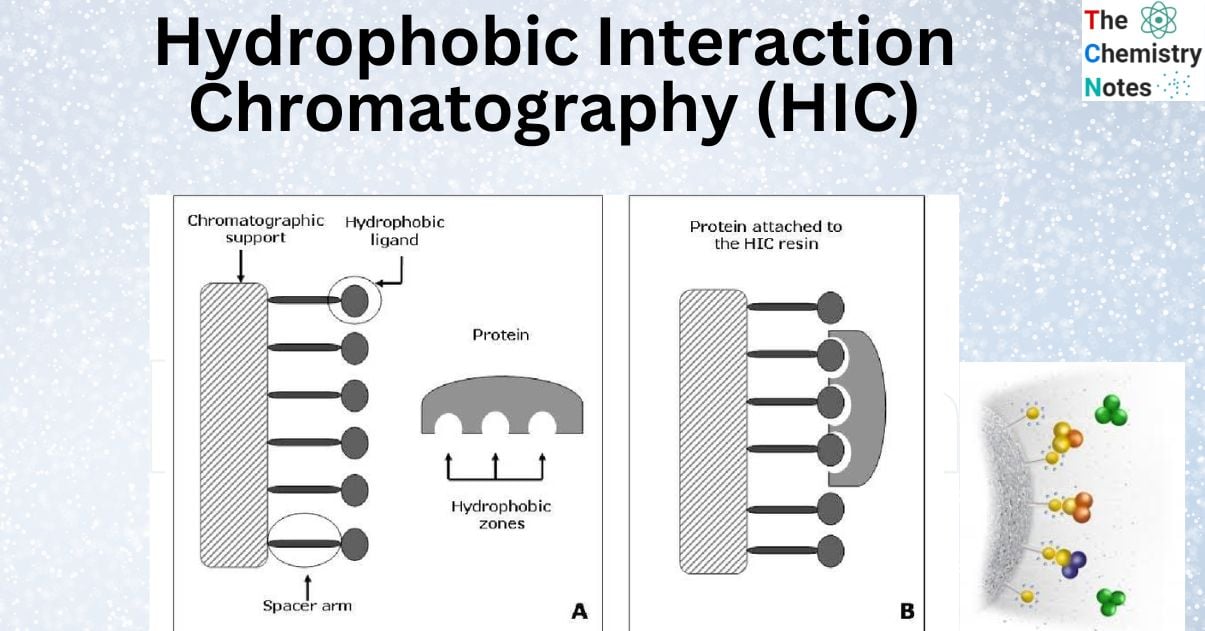
Principle of Hydrophobic interaction chromatography
- Hydrophobic interaction chromatography involves introducing the sample protein molecules into a column that contains a high-salt buffer.
- By lowering sample molecule solvation and exposing their hydrophobic regions, the salt encourages interaction between the hydrophilic and hydrophobic portions of the protein and the medium.
- When dissolved in an aqueous solution, hydrophobic proteins will interact or form associations with one another. Several biological interactions, including protein folding, interactions between proteins and their substrates, and the transport of proteins across cellular membranes, are based on this self-association. In order to bind to hydrophobic ligands on chromatographic adsorbents, macromolecules that include hydrophobic areas take use of these regions. The interaction takes place in a setting that encourages hydrophobic interactions, such as an aqueous solution with a lot of salt.
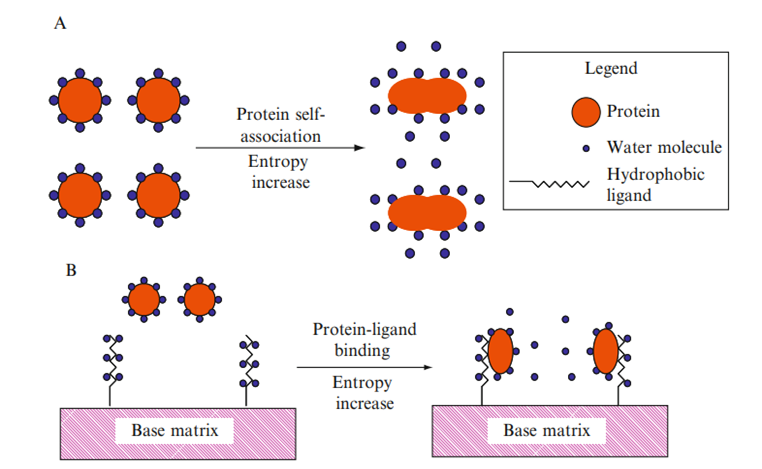
Proteins will aggregate or self-associate to reach a state with the lowest thermodynamic energy. Water molecules surround each individual macromolecule in highly organized arrangements before self-attachment. A net rise in the environment’s entropy promotes the self-association of nonpolar molecules (like proteins) in the polar solvent. The protein’s overall surface area of hydrophobic sites exposed to the polar solvent decreases during the aggregation process, leading to a less structured (higher entropy) situation that is the preferred thermodynamic state.
The connection (association) between hydrophobic ligands coupled to an adsorbent and the target proteins is caused by the same idea. When compared to the situation where there is no connection between the protein and the adsorbent, the association or hydrophobic interaction between the protein and the hydrophobic ligand is predominantly caused by an increase in total entropy.
The hydrophobicity of the molecules has an inverse relationship with the quantity of salt required to induce binding. As a result, the sample molecules can be removed using a decreasing salt gradient in order of increasing hydrophobicity. Washing with a diluted buffer or water can efficiently desorb bound protein molecules.
General considerations for hydrophobic interaction chromatography
The ligand, matrix, salt concentration, pH, and temperature are among the general considerations for hydrophobic interaction chromatography.
Ligand: The type of immobilized ligand determines a protein’s adsorption behavior. Straight-chain alkyl ligands often exhibit a hydrophobic nature, whereas aryl ligands have a mixed-mode behavior that allows for both aromatic and hydrophobic interactions.
The extent of substitution: With a greater degree of immobilized ligand substitution, the protein binding capability rises. Although the affinity of the interaction rises with a high level of ligand replacement, the binding capacity remains constant. Due to the multiple points of attachment, it is challenging to elute proteins bound under these circumstances.
Matrix: Cross-linked agarose and synthetic copolymer materials are the hydrophilic carbohydrates that are most frequently employed as supports. Even if the ligands may be the same, the selectivity between various supports won’t be the same. When switching from one medium to another, alter the adsorption and elution settings to obtain comparable outcomes.
The concentration of salt: In HIC, ligand-protein interactions are facilitated by the addition of structured salts to the equilibration buffer and sample. The quantity of bound protein increases along with the salt concentration, as does the possibility of protein precipitation at greater ionic strengths.
pH: HIC mobile phases are commonly buffered with sodium or potassium phosphate and have neutral pH values between 5-7. In general, when the charge of the protein increases owing to acidic group titration, the intensity of the interaction between proteins and medium diminishes with rising pH. Between proteins, this impact might differ. As a result, media selectivity and protein binding levels can both be affected by pH. In contrast, over moderate ranges, pH variations have no appreciable impact.
Temperature: The temperature enhances the affinity of hydrophobic contacts. Protein solubility, interaction with the HIC matrix, and structural characteristics are all influenced by temperature.
The procedure of Hydrophobic interaction chromatography
- Hydrophobic interaction chromatography media, Alkyl, or aryl ligands are linked to an inert, porous matrix makeup, which is subsequently arranged in a packed bed configuration inside a chromatography column.
- The pores and spaces between the matrix’s particles are filled with a slightly salty buffer. Salts like 1-2 M ammonium sulfate or 3 M sodium chloride are typically utilized, and they are chosen to maximize the critical interaction between the protein sample and the medium while decreasing that of other, less hydrophobic proteins (impurities).
- Non-bound proteins are removed from the column by washing.
- To start eluting proteins, the salt concentration is gradually reduced. Proteins can be eluted differently by manipulating salt gradients; the proteins with the lowest hydrophobicity are eluted first.
- Using a salt-free final wash aid in releasing firmly bound proteins. The desorption of the attached proteins can be further accelerated by buffer additives. Detergents, chaotropic (“salting-in”) salt solutions, and water-miscible alcohols are examples of additives.
- Rarely are stronger conditions needed to completely dissolve all bound proteins, such as 0.5-1.0M sodium hydroxide, 70% ethanol, or 30% isopropanol.
Mechanism/Theories Behind Hydrophobic Interaction Chromatography
Theory of Salting Out
The hydrophilic amino acids enable proteins to connect with the hydrogen molecules in the water, whereas the hydrophobic amino acids create protected hydrophobic regions when folding in an aqueous solution, according to the salting out the theory. If enough hydrophilic regions are found on the surface of the protein molecules, the likelihood that the protein will dissolve in water will rise. The protein molecules interact freely with one another in a solution when the strength of the solvent-solute interactions outweighs the strength of the protein-protein interactions, which causes the protein molecules to aggregate and finally precipitate out of the solution. As a result, adding salt to the solution decreases the solubility of certain proteins to variable degrees.
Theory of Thermodynamics
According to this idea, which is based on the second law of thermodynamics, the interaction between hydrophobic molecules is an entropy-driven process. A change in entropy controls the hydrophobic contact between two or more non-polar molecules in a polar solvent solution. However, by adjusting the temperature or the solvent polarity, these interactions may be changed.
As a result, there will be an increase in the degree of order of the solvent molecules around the hydrophobic molecule when a non-polar molecule interacts with a polar solvent, such as water. This will result in a drop in entropy and a general improvement in the Gibbs energy, so long as enthalpy does not rise noticeably.
In contrast, when two or more non-polar molecules are present in a polar environment, the hydrophobic surfaces of the macromolecules are shielded from the polar environment and the hydrophobic molecules spontaneously combine. The highly structured solvent molecules that surround the exposed surface of the hydrophobic molecules will be moved toward the solvent’s bulk of less structured molecules. Entropy rises as a result, whereas the Gibbs energy falls. In such circumstances, hydrophobic contact turns into a process that is thermodynamically advantageous.
Van der Waals Theory
According to this idea, van der Waals forces between proteins and immobilized ligands are what drive the hydrophobic interaction in Hydrophobic interaction chromatography because they grow as the ordered structure of water rises in the presence of salting out salts.
Applications of HIC and Recent advancements
- Hydrophobic interaction chromatography is a well-known purification technique used in the synthesis of proteins and antibodies in both scientific and commercial settings.
- It has mostly been employed for the removal of pollutants from processes, such as host cell proteins, as well as contaminants associated with products, such as aggregates.
- Much of the recent progress in HIC chromatography in terms of higher throughput and improved selectivity or resolution has been driven by the process economics and needs of high product purity and quality.
- To speed up the process development process, high throughput screening (HTS), design of experiments, and platform method have been used. The increase in throughput was made possible by improved resins with greater binding power, dual salt load conditioning, and flow-through operation.
- The use of buffers and bed volumes are decreased through hydrophobic interaction membrane filter chromatography, and cycle times may be shortened, thereby increasing process throughput.
- Enhancements to selectivity and/or resolution have been made by the introduction of solvent additives and the modification of operational parameters like temperature.
The Pros and Cons of Using HIC
Pros
- Hydrophobic interaction chromatography is most frequently used to distinguish aggregated protein species from a more advantageous monomeric form. Compared to other types of chromatography, such as ion exchange and affinity.
- HIC frequently exhibits better selectivity for the removal of aggregate species.
- This could potentially offer better selectivity for the elimination of unwanted protein variants or misfolded forms.
Cons
In order to establish enough hydrophobic contact between the protein and the adsorbent, the use of Hydrophobic interaction chromatography frequently necessitates the use of high salt concentrations. It may be expensive to generate buffers with high salt concentrations, and it may also be expensive to properly dispose of them (depending on the existing environmental disposal requirements). The use of other chromatographic techniques may be preferable in certain circumstances.
References
- Hofstee BH and Otillio NF (1978). Non-ionic adsorption chromatography of proteins. J Chromatogr 159, 57–69. PMID: 649758
- Jennissen HP (1978). Multivalent interaction chromatography as exemplified by the adsorption and desorption of skeletal muscle enzymes on hydrophobic alkyl-agaroses. J Chromatogr 159, 71–83. PMID: 418077
- Jennissen HP and Heilmeyer LM, Jr. (1975). General aspects of hydrophobic chromatography. Adsorption and elution characteristics of some skeletal muscle enzymes. Biochemistry 14, 754–760. PMID: 163642
- https://www.bio-rad.com/en-np/applications-technologies/introduction-hydrophobic-interactionchromatographyhic?ID=MWHB53MNI#:~:text=Hydrophobic%20interaction%20chromatography%20(HIC)%20separates,operate%20under%20less%20denaturing%20conditions.
- https://www.news-medical.net/life-sciences/Hydrophobic-Interaction-Chromatography-(HIC).aspx
- https://www.chromatographyonline.com/view/hydrophobic-interaction-chromatography-proteins
- Recent Advancement in Application of Hydrophobic Interaction Chromatography for Aggregate Removal in Industrial Purification Process: Yuefeng Lu, Brian Williamson and Ronald Gillespie: http://dx.doi.org/10.2174/138920109788488897.
- https://info.gbiosciences.com/blog/the-basics-of-hydrophobic-interaction-chromatography
- Theory and Use of Hydrophobic Interaction Chromatography in Protein Purification Applications- Justin T. McCue, https://doi.org/10.1016/S0076-6879(09)63025-1.
