Hydrometallurgy is the process of extraction of metals from their ores. It is an extractive metallurgy technology that involves the use of aqueous chemistry to recover metals from ores, concentrates, and recycled or residual materials. Hydrometallurgy will play an increasingly important role in the extraction of precious metals from sulfide minerals in the future. It is unique in that it is applied to low-grade ores that cannot be profitably beneficiated.
Interesting Science Videos
Principles of hydrometallurgy
When metals are chemically processed in an aqueous environment, the technology used is known as hydrometallurgy, and it consists of three separate stages:
- The metal of interest must first be transported from the solid feed material (ores, concentrates, etc.) into an aqueous solution.
- This metal-containing solution (or solutions produced from it) must subsequently be concentrated and purified.
- The metal must subsequently be retrieved from the purified solution in solid form.
Different processes involved in hydrometallurgy
Leaching
Leaching is a fundamental extractive operation in hydrometallurgical processing that involves the transfer of a metal of interest from naturally occurring minerals into an aqueous solution. In basic terms, it involves the selective dissolution of precious minerals by exposing the ore to an active chemical solution known as a leach solution.
Leaching is the process of extracting metal from metal-bearing materials that come into contact with a valuable metal using aqueous solutions. It is the process of bringing aqueous solutions containing a lixiviant into contact with a substance containing precious metal. The lixiviant in the solution might be acidic or basic. The lixiviant type and concentration are generally regulated to allow some degree of selectivity for the metal or metals to be recovered.
To optimize the rate, extent, and selectivity of dissolution of the desired metal component into the aqueous phase, the lixiviant solution conditions vary in terms of PH, oxidation-reduction potential, the presence of chelating agents, and temperature. Certain metals can be extracted selectively using chelating agents.
Factors affecting the choice of leaching agents
The choice of leaching agents depends on:
- The chemical and physical properties of the substance to be leached
- The price of the reagent.
- The corrosive effect of the reagent and the construction materials
- The leaching agent’s selectivity for the intended ingredient to be leached.
- The ability to be renewed.
Common leaching agents
Water
Some compounds, such as CuSO4, ZnSO4, and the majority of alkali metal compounds, dissolve quickly in water. Some low-grade copper sulfide ores progressively convert into water-soluble sulfate.
Mineral acids, particularly sulphuric acids, are the most commonly used leaching agents.
Bases
Several bases, such as NaOH solution or NH4OH, are commonly used in numerous leaching procedures. Bauxite is leached under pressure using a hot concentrated NaOH solution, whereas ammonia solution is utilized in the leaching of native copper, copper ores, NiS, and Cu2S.
Aqueous salt solutions
The dissolution of gold during its extraction from veins in silica rock is the most prominent example of a salt solution acting as a leaching agent. A solution of NaCN dissolves gold. The reaction is,
4 Au(s) + 8 NaCN(aq.) + O2(g) + 2 H2O(aq.) ⇋ 4 NaAu(CN)2 (aq.) + NaOH(aq.)
Separation of leach liquors
Almost all hydrometallurgical processes include the leaching of particles in order to dissolve valuable components. Before generating the final product, solid-liquid separation is frequently performed. In this stage, the leach liquor solution is separated from the solid residues using one or more methods. Common methods of separation of leach liquors involve:
Washing
This process is based on the density difference between metallic ore and contaminants. When the ore is processed with a stream of running water, lighter impurities are washed away, leaving heavier ore particles behind.
Filtration
The separation of a suspension into a solid filter cake and a liquid filtrate by passing it through a permeable filtering material is known as filtration. The qualities of the suspension (e.g., size distribution, concentration), filtering materials (for example, the width and shape of pores), as well as the forces, applied to the suspension are important factors to be considered during this process.
Thickening
In leaching operations, thickening is the separation of a slurry or solid-liquid mixture into a dense slurry containing the majority of the solids and an overflow of liquor. The solids in a suspension settle in a tank due to gravity and produce a thick pulp.
The pulp and clear liquid at the tank’s top can be removed continuously or occasionally.
Settling
In this method of solid-liquid separation, the leachate obtained after leaching is neutralized, and the neutralized slurry is separated and removed by adding flocculants
(A flocculant is a chemical that can be added to water to help colloids and other suspended particles combine to generate heavier particles)
Important factors to be considered during the leaching process
Particle size
The ore or concentrate particles must be tiny enough for the valuable metals contained within them to be physically exposed to the leach solution. The rate of leaching is affected by the degree of exposure. In general, the rate of leaching increases as particle size decreases.
Rates of diffusion
The rate of diffusion of reactants or products can influence the rate of a leaching mechanism. When a species in the solution phase diffuses slowly to and from the mineral surface, enhancing the degree of agitation of the solution, it increases the frequency at which the species diffuses.
The rate of a chemical reaction.
In some cases, the rate at which leaching processes occur at the mineral surface dictates leaching kinetics. The degree of exposure of the valuable metal can be increased, the leaching system’s temperature or pressure can be raised, or a catalyst can be used to speed up the chemical reaction rate.
The concentration of the leaching agent
The rate of leaching increases as the concentration of the leaching agent increases.
Insoluble substances
If an insoluble reaction product is generated during leaching, its rate will be determined by the nature of the product. The rate of leaching will be considerably reduced if it forms a non-porous layer. If the solid product is porous, the rate will be affected only slightly or not at all.
Types of leaching process
In situ leaching
It is also referred to as solution mining.
It is a mining method that involves drilling boreholes into a deposit to recover minerals such as copper and uranium. The procedure begins with the drilling of holes into the ore deposit. The leaching solution is poured into the deposit and comes into contact with the ore. The dissolved ore-containing fluid is then pumped to the surface and treated.
The solvent is fed into the ore via a series of pipelines bored into the ground. The resulting liquor is extracted using a variety of pipe shape drills. The solvent flows down and is penetrated into the ore body by these pipe-shaped drills for leaching. The solute is leached as the solvent flows through the pipe-shaped drilling and ore body.
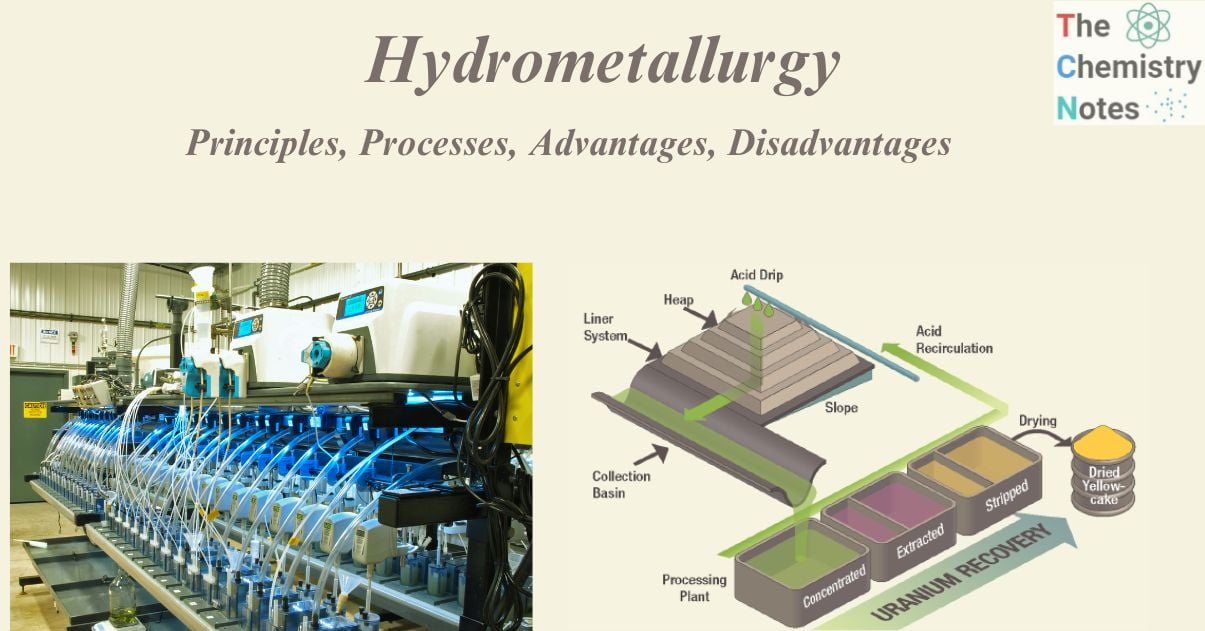
Heap leaching
Crushed (and often agglomerated) ore is placed in a heap lined with an impermeable layer in heap leaching procedures. The leach solution is sprayed on top of the heap and allowed to trickle downhill through it. The heap design typically includes collection sumps that allow the “pregnant” leach solution (solution containing dissolved valuable metals) to be supplied for further processing. Gold cyanidation is one example, in which pulverized ores are extracted with a solution of sodium cyanide, which dissolves the gold in the presence of air, leaving behind the nonprecious residue.
Dump leaching
Dump leaching combines heap and in-situ leaching properties. Depending on the dump location, an impermeable layer may or may not be employed in a dump leach. The ore is dumped to allow similar processing to heap leaching, but the geological conditions of the area allow a valley or pit to act as the sump.
Agitation leaching
A time-consuming procedure in which soil is slurried with fluid extraction. When equilibrium is reached between the metals on the soil surface and the metal contained in the solution, the solubilization of metal in the soil slows and extraction is considered complete.
Vat leaching
Vat leaching is the process of interacting material with leach solution in big tanks or vats after it has been size reduced and classified. Agitators are frequently used in vats to maintain solids suspended in the vats and increase solid-liquid interaction. Prior to further processing, the leached particles and pregnant solution are normally separated after vat leaching.
Autoclave leaching
Autoclave reactors are used for reactions at higher temperatures, which might increase the rate of the reaction. Similarly, autoclaved allow the use of gaseous reagents in the system.
Factors affecting the viability of leaching operation
The degree of dissolution.
The dissolution of the value metals must be as complete as possible for a high degree of metal extraction from an ore, which is the fundamental consideration to take into account in any leaching process.
Leaching selectivity
The selectivity of the dissolving reactions is the second aspect that influences the viability of leaching. This is significant since all ores contain minerals other than those containing the metal of interest.
The selectivity of the leaching reactions dictates the extent to which this occurs and, as a result, the purity of the metal-bearing solution produced by the leach.
The capital cost of the leach apparatus
The capital cost of leaching equipment can vary greatly, especially when the materials of construction are considered, which is dependent on the needed working conditions.
The price of leach solutions
The unit costs of the various chemicals and the quantities utilized influence the cost of the leaching process.
Solution concentration and purification
Following leaching, the leaching liquid must generally be concentrated to recover the metal ions. Furthermore, some unwanted metals may have been dissolved during the leaching process. To remove the unwanted components, the solution is frequently filtered. The following processes are used for solution concentration and purification:
Precipitation
Precipitation is the selective elimination of a specific metal compound or the removal of a significant contaminant by precipitation of one of its components. Chemical precipitation is the primary method for recovering or removing metals from a solution. Chemical precipitation is one of the most extensively used techniques for heavy metal removal from inorganic effluents in industry. It primarily involves the use of chemicals (precipitants) to convert a soluble substance into an insoluble form (insoluble precipitates of heavy metals like hydroxide, sulfide, carbonate, and phosphate). Heavy metals can be easily removed once they have precipitated to form solids.
Cementation
A form of precipitation, cementation is a heterogeneous process in which ions are reduced to zero valence at a solid metallic contact. It is the extraction of metals from a solution based on an electrochemical reaction between the cementing metal and the precipitated metal’s ion. The precipitation of the metal is followed by a change in its concentration in the solution, and hence by a change in its potential. When the equilibrium values are reached, the process comes to an end.
Electrowinning
Electrowinning is the electrodeposition of metals from their ores that have been immersed in a solution. It’s sometimes referred to as electro-extraction. Because of their high electro-potential values, it is most typically employed to recover metals such as gold, silver, copper, and zinc.
Current is carried from an inert anode through a liquid leach solution containing the metal in the electrowinning process, allowing the metal to be recovered as it deposits on the cathode.
Electrowinning cells provide companies with a low-cost choice with improved efficiency. Furthermore, electrowinning yields exceedingly clean goods.
Solvent extraction
Metal is extracted from one phase to another using an extractant and a diluent combination. Because the major element (diluent) is some form of oil, this mixture is typically referred to as “organic” in solvent extraction.
The pregnant leach solution (PLS) is emulsified with the stripped organic and then separated. Metal will be transferred from the PLS to the organic. The end outcome will be a loaded organic and a raffinate stream. When electrowinning is used, the loaded organic is emulsified with a lean electrolyte and allowed to separate. Metal will be transferred from the organic to the electrolyte.
The streams that arise will be a stripped organic and a rich electrolyte. The organic stream is recycled via the solvent extraction process, whereas the aqueous streams are recycled via the leaching and electrowinning processes.
Ion exchange
Ion exchange has traditionally been used to purify water and remove metal pollutants from dilute waste streams. Its application in eliminating trace metallic contaminants from hydrometallurgical process streams has recently expanded significantly. It is also employed as a primary recovery and concentration unit operation for particular commodities, where both technical and cost advantages become apparent. The solution is exchanged for cations or anions using chelating agents, natural zeolite, activated carbon, resins, and liquid organics coated with chelating agents. The chemicals used and the impurities present influence selectivity and recovery.
Metal recovery
The final step in a hydrometallurgical process is metal recovery. Metals suitable for sale as raw materials are frequently produced directly during the metal recovery stage. However, if ultra-high purity metals are to be produced, additional refining may be required. Electrolysis, gaseous reduction, and precipitation are the three main types of metal recovery techniques. Copper, for example, is a primary target of hydrometallurgy since it is easily obtained through electrolysis. Cu2+ ions decrease at low potentials, leaving other contaminating metals like Fe2+ and Zn2+ behind.
Electrolysis
Electrowinning and electrorefining are two processes that use electrodeposition of metals at the cathode and either metal dissolution or a competitive oxidation reaction at the anode to recover and purify metals.
Precipitation
In hydrometallurgy, precipitation is the chemical precipitation of metals and their compounds or impurities from aqueous solutions. Precipitation occurs when a particular species’ limit of solubility is exceeded through reagent addition, evaporation, pH change, or temperature manipulation.
Advantages of hydrometallurgy
- Hydrometallurgy is presently employed to create over 70 metallic elements, and it involves selective separation in battery recycling, which results in salt extraction. After desorption of the adsorbed ions, ion exchange leads to a variety of separation and recovery procedures for metals at low concentrations.
- Hydrometallurgy is capable of extracting complicated and low-grade ores.
- Hydrometallurgy has a modest investment cost.
- Hydrometallurgy is significantly more environmentally friendly.
- Greater control over each stage of the process leads to the recovery of valuable by-products. Material handling is also simplified.
- A hydrometallurgical method eliminates the need for coke, an increasingly expensive reducing agent.
- Liquor waste from the last recovery process may be recycled.
Disadvantages of hydrometallurgy
- Hydrometallurgy uses a huge amount of water, which has a greater potential for contamination.
- There are difficulties in solid-liquid separation;
- impurities problems may arise throughout the purification process during hydrometallurgy.
- More time is required for high metal recovery.
Advantages of hydrometallurgy over pyrometallurgy
- Low-temperature processing, low handling cost of leaching products, and the ability to treat low-grade ores in hydrometallurgy make leaching superior to high-temperature smelting.
- Sulfides are burned off in traditional pyrometallurgical smelting, releasing SO2 gas into the atmosphere. In comparison to pyrometallurgy, hydrometallurgy releases only a fraction of the gases into the atmosphere.
- Hydrometallurgy is more environmentally friendly than pyrometallurgy.
- Hydrometallurgy is suitable for lean and complicated ores. With the steady depletion of rich ore resources, traditional pyrometallurgical processes for metal extraction are becoming increasingly challenging in many instances.
- A hydrometallurgical process can begin on a modest scale and scale increase as needed. Because of the need for process economy, pyrometallurgical processes must typically be planned as large-scale operations.
References
- https://www.britannica.com/technology/hydrometallurgy
- https://www.chemeurope.com/en/encyclopedia/Hydrometallurgy.html.
- https://chem.libretexts.org/Courses/University_of_Missouri/MU%3A__1330H_(Keller)/23%3A_Metals_and_Metallurgy/23.3%3A_Hydrometallurgy#:~:text=Hydrometallurgy%20involves%20the%20use%20of,metals%20like%20gold%20and%20silver.
- http://archive.nitjsr.ac.in/course_assignment/MME09MT%201403%20PEMUNIT%20PROCESSES%20IN%20HYDROMETALLURGY%20LEACHING,%20PURIFICATION%20OF%20LEACH%20LIQUOR.pdf.
- https://repository.nwu.ac.za/bitstream/handle/10394/9626/Smit_DS_Chapter_2.pdf?sequence=3&isAllowed=y
- https://www.purolite.com/dam/jcr:3fc1fd51-bef0-4406-803f-e85e3bb83160/hydrometallurgy-applications-guide.pdf
