Hydrocarbons are organic chemical compounds entirely made up of hydrogen and carbon atoms. Hydrocarbons are naturally occurring substances that serve as the foundation for crude oil, natural gas, coal, and other vital energy sources. They are highly combustible, emitting carbon dioxide, water, and heat when burned. As a result, hydrocarbons are an excellent source of fuel.
Hydrocarbons have the chemical formula CxHy. In these compounds, the carbon atoms bind together to form the compound’s framework, and the hydrogen atoms attach to them in a variety of ways. Despite the fact that they are made up of only two types of atoms, hydrocarbons come in a broad range because they can have simple or very complex structures.
Many hydrocarbons have been discovered in plants, animals, and their fossils; others have been synthesized in the laboratory. Natural gas, acetylene, propane, butane, and the primary components of gasoline, diesel fuel, and heating oil are all examples of hydrocarbons that we all use every day. Hydrocarbons include the well-known polymers polyethylene, polypropylene, and polystyrene. Differences in the bonding between carbon atoms allow us to distinguish between different types of hydrocarbons.
Interesting Science Videos
Properties of Hydrocarbons
- The melting and boiling temperatures of hydrocarbon molecules are influenced by their size.
- Certain hydrocarbons are gases at room temperature, whereas others are liquids or solids.
- They are made up of strong bonds between carbon and hydrogen.
- Hydrocarbons exhibit strong attractive molecular forces.
- Due to their lower density than water, hydrocarbons are able to float on water’s surface.
Classification of Hydrocarbons
Based on their sources and qualities, chemists in the nineteenth century categorized hydrocarbons as aliphatic or aromatic. Aliphatic hydrocarbons (from Greek aleiphar, “fat”) were produced from the chemical breakdown of fats or oils. Aromatic hydrocarbons were a class of related compounds derived from the chemical breakdown of certain pleasant-smelling plant extracts. Modern terminology uses the terms aliphatic and aromatic hydrocarbons, but the substances that they describe are distinguished by structure rather than origin. So, Hydrocarbons are broadly classified into two categories depending upon the nature of their carbon skeleton (i.e. on the basis of their structure). These are:
- Acyclic or open chain or aliphatic compounds
- Cyclic or closed chain or ring compounds
Acyclic or open-chain, or Aliphatic compounds
Aliphatic compounds or aliphatic hydrocarbons are hydrocarbon compounds that have carbon and hydrogen connected to one other in straight chains by single, double, or triple bonds, branching chains. Aliphatic chemicals can be saturated, such as hexane, butane, propane, ethane, and so on, or unsaturated, such as propene, ethyne, butyne, and so on. Aliphatic is a phrase derived from the Greek word meaning fat or oil. These molecules are known as aliphatic compounds because they were originally thought to be derived from fats and oils. Methane is the simplest aliphatic hydrocarbon.
Properties of aliphatic hydrocarbons
- The vast majority of aliphatic chemicals are combustible. They are frequently employed as fuel sources, such as methane, propane, ethylene, acetylene, and so on.
- Aliphatic compounds’ melting points rise with size but in an unusual way.
- The hydrocarbons are nonpolar, hence they are insoluble in polar solvents like water. However, they dissolve in non-polar solvents such as benzene and diethyl ether.
Aliphatic hydrocarbons are further divided into saturated and unsaturated hydrocarbons.
Saturated hydrocarbons
In saturated hydrocarbons, single bonds hold carbon-carbon and carbon-hydrogen atoms together. These single-bonded molecules are the most basic hydrocarbons. They have Sp3 hybridized carbon atoms but no Sp2 or Sp hybridized carbon atoms.
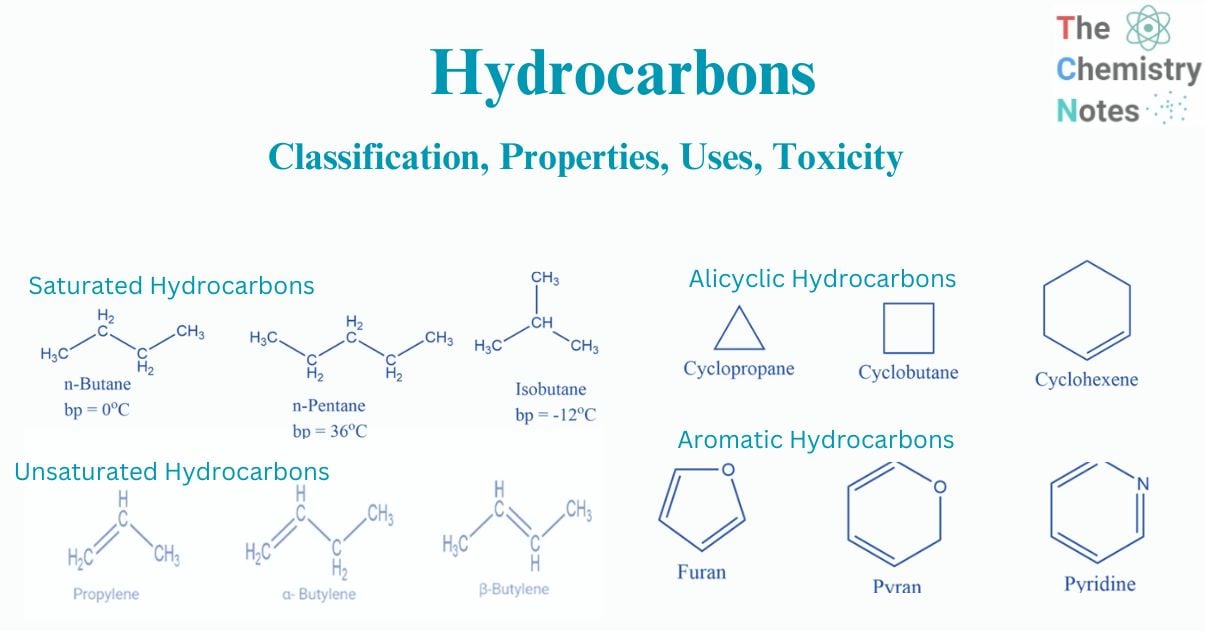
Saturated compounds are hydrocarbons with only carbon-carbon single bonds. These are the most frequent type of hydrocarbon. In other words, as carbon atoms are saturated with hydrogen atoms, carbon is connected with hydrogen atoms as possible. The vast majority of saturated hydrocarbons are alkanes, which are open chain hydrocarbons with a single carbon-carbon link. Typically, the bond is covalent. Because of their inert nature, these compounds do not easily react with acids, bases, or other reagents. The group of saturated hydrocarbons includes all alkanes. These can be branched, like isobutane, or straight chain, like n-butane.
Alkane
Alkanes are the most basic organic compounds, made up of only carbon and hydrogen. They are represented by the general formula CnH2n+2. As their carbon skeleton is completely saturated with hydrogens, they are also known as saturated hydrocarbons. Since alkanes are made up of strong C-C and C-H covalent bonds, they are chemically inert.
Properties of alkane:
- Methane, ethane, propane, and butane are present in the gas state. Higher alkanes are wax-like solids, whereas C5-C17 alkanes are colorless liquids.
- They are nonpolar compounds, so they are insoluble in polar solvents. They are soluble in nonpolar solvents such as benzene and carbon tetrachloride.
- With increasing molecular weight, the boiling point of alkanes rises. The boiling points of straight-chain alkanes are higher than those of branched alkanes.
Unsaturated hydrocarbons
These compounds are made up of a single, double, or triple bond between carbon atoms. Triple-bonded compounds are known as alkynes, while double-bonded ones are known as alkenes. The general formula for alkenes is CnH2n, whereas the general formula for alkynes is CnH2n-2. Unsaturated hydrocarbons include at least one carbon-carbon double (pi) bond or triple bond.
Alkene
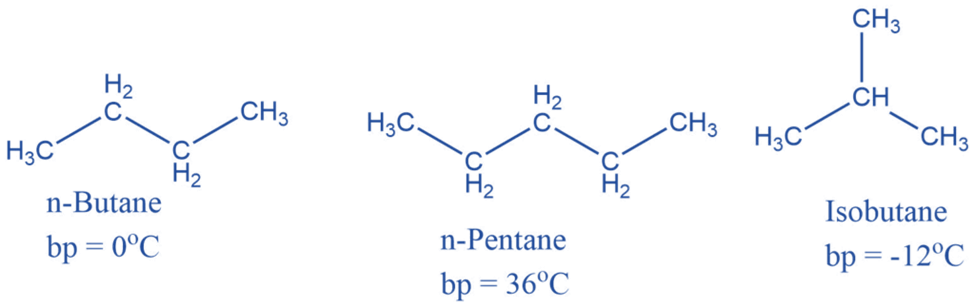
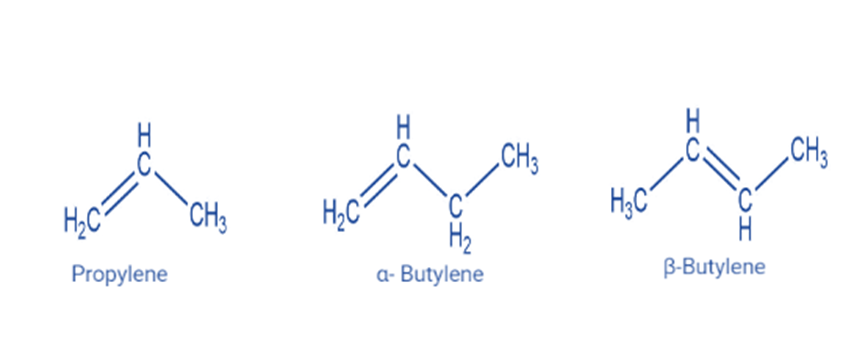
Alkenes are unsaturated hydrocarbons with carbon-carbon double bonds. An alkene has the generic formula CnH2n, where n is an integer number. Alkenes have two fewer hydrogen atoms than alkanes.
Properties of alkene
- Lower alkenes are gases, while higher alkenes are liquids. Alkenes with more than 18 carbon atoms in the molecule are solids.
- Except for ethene, they are colorless and odorless. Ethene has a mildly pleasant aroma.
- Alkenes are water-insoluble however, they are easily soluble in organic solvents.
- In general, as the molecular weight of a substance increases in a homologous series, so do its melting point, boiling temperature, and specific gravity.
- Compared to the corresponding alkanes, alkenes are less volatile.
Alkyne
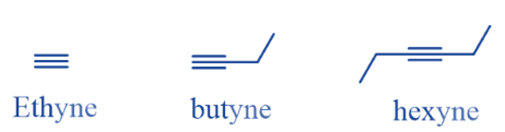
The alkynes are unsaturated hydrocarbons with one triple bond They are represented by the general formula CnH2n-2. The triple bond in alkyne is known as the ‘acetylenic bond’. There are numerous alkynes found in nature. Ethyne (C2H2) is the first member of the alkyne family, Which consists of two carbon bonded together by triple bonds.
Properties of alkynes
- Alkynes are non-polar organic molecules.
- They are water insoluble but are soluble in non polar solvents.
- They have a lower density than water.
- Alkynes have very low melting and boiling points.
- The melting and boiling points increase as the number of carbons increases.
- Terminal alkynes are more acidic than other hydrocarbons.
Cyclic hydrocarbons
In chemistry, cyclic compounds are molecules with atoms bound to one other to form a ring structure. At least three atoms must be linked together to form a ring. These cyclic compounds are further divided into homocyclic and heterocyclic compounds.
Homocyclic compounds
Homocyclic compounds are cyclic compounds with ring members composed of the same element. Homocyclic compounds are organic molecules that exclusively include carbon atoms. These are also referred to as carbocyclic compounds or carbocycles. Homocyclic compounds are further divided into alicyclic and aromatic.
Alicyclic
The cyclic aliphatic compound is one in which the carbon chain forms a ring around itself. They may also be saturated and unsaturated. They are cyclic compounds in which the carbon atoms are linked together to form one or more rings. They do not have aromatic properties. They might be saturated or unsaturated. They are categorized as cycloalkane, cycloalkene, and cycloalkyne, just as open-chain compounds.

A cycloalkane is a cyclic hydrocarbon that contains only single carbon-carbon bonds. Cycloalkanes, like other alkanes, are saturated molecules. The typical formula for cycloalkanes is CnH2n. The most basic cycloalkane is cyclopropane, which has a three-carbon ring.
Properties of alicyclic compounds
- They might be saturated or unsaturated.
- They don’t have an aromatic character.
- In a ring, three or more carbon atoms are bonded together.
- Single bonds with two electrons or double or triple bonds with four or six electrons can exist between nearby atoms.
- Alicyclic compounds with three or four-carbon atom rings are less stable than compounds with higher rings.
- They have a greater melting and boiling point.
- They have a higher density.
- Carbon atoms are in close proximity in their ring structure.
- They are quite reactive.
- They have angle strain.
- The first four classes exist as gases at room temperature.
- They are not soluble in water.
- Their molecules are destroyed when they are burned.
Aromatic compounds
The word “aromatic” derives from the word “aroma,” which refers to a pleasing odor, therefore aromatic chemicals have a pleasant odor. A cyclic, planar molecule having a ring of resonance bonds is known as an aromatic compound. Such structures are more stable than regular rings. Arenes are another name for these hydrocarbons. Because the majority of aromatic hydrocarbons contain benzene rings, they are referred to as benzenoids. Some aromatic hydrocarbons lack benzene rings and are hence referred to as Non-Benzenoids. As a result, aromatic compounds are broadly divided into two categories: benzenoids (those with a benzene ring) and non-benzenoids (those without a benzene ring).
Benzenoid
Benzenoid substances have at least one benzene ring in their chemical structure. A benzene ring is a cyclic structure made up of six carbon atoms. It is made up of three pi bonds (double bonds) and three sigma bonds organized in a different configuration. As a result, this pattern is known as a conjugated pi system. According to the number of benzene rings connected inside their structures, benzenoid compounds are classified as monocyclic, bicyclic, or tricyclic.
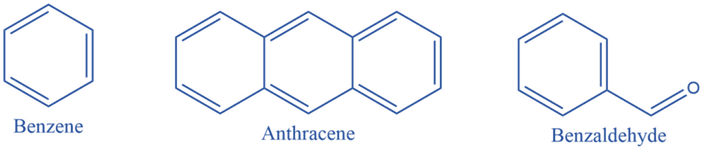
Non-benzenoids system
Non-benzenoid compounds are aromatic molecules that lack benzene rings in their chemical structure. Despite the lack of a benzene ring, these molecules have a conjugated pi system. Because of the existence of a conjugated pi system, these molecules have an aromatic character. This conjugated pi system offers extra stability to the molecule. Examples include azulenes, Oxaazulanones, Pentafulvene, Tropones and Tropolones, and more.
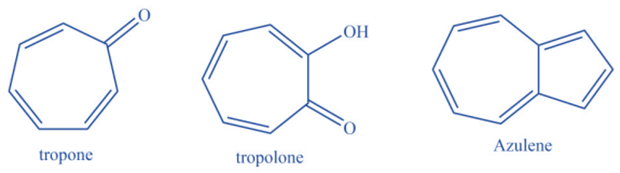
Properties of aromatic compounds
- They are cyclic compounds with five, six, or seven-membered rings that have a flat (planar) structure.
- They are highly unsaturated compounds that show electrophilic substitution reactions and are resistant to addition reactions.
- They have proven to be more stable than expected. They have lower heats of hydrogenation and heats of combustion than expected.
- Aromatic compounds have a lower reactivity than aliphatic substances.
- The percentage of carbon in aromatic compounds is higher than in aliphatic compounds. As a result, they burn with a sooty flame.
- Aromatic hydroxy compounds (phenols) are acidic, whereas alcohols are neutral.
- Aromatic amines (anilines) have a lower basicity than aliphatic amines.
- In comparison to aliphatic molecules, aromatic compounds quickly undergo sulphonation and nitration reactions.
- Aromatic compounds have a delocalized Pi cloud that is uniformly distributed on the ring system. The electron cloud must contain (4n+2)∏ electrons, i.e. they must fulfill Huckcl’s (4n + 2)∏ electron rule, where n = integer 0, 1, 2, 3, and so on. This is referred to as Huckel’s rule.
Heterocyclic compounds
Heterocyclic organic compounds are compounds with at least one heteroatom (atom other than carbon) in the cyclic ring structure. Nitrogen (N), oxygen (O), and sulfur (S) are the three most prevalent heteroatoms. Heterocyclic compounds are commonly found in plants and animal products, and they are an essential element of more than half of all natural organic compounds. Some examples of natural heterocyclic compounds include alkaloids, natural colors, medicines, proteins, enzymes, and others.
The heterocyclic compounds may be divided into two types based on their structural and electronic arrangement.
- Aliphatic heterocyclic compounds
- Aromatic heterocyclic compounds
Aliphatic heterocyclic compounds
Saturated heterocycles are aliphatic heterocycles that do not contain double bonds. The ring strain has the greatest influence on the characteristics of aliphatic heterocycles. Aziridine, Ethylene Oxide, Thiirane, Oxetane, Azetidine, Thietane, Tetrahydrofuran (THF), Dioxane, Pyrrolidine, Piperidine, and others are examples of aliphatic heterocyclic compounds.
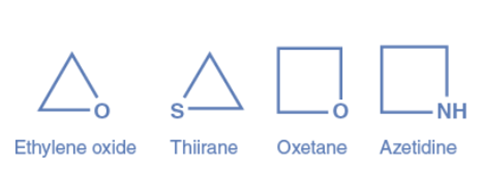
Aromatic heterocyclic compounds
Aromatic heterocyclic substances are benzene analogs.
Aromatic heterocyclic compounds must also adhere to Huckel’s rule (i.e. aromatic compounds must be cyclic in nature, have planar geometry due to conjugate double bonds, and have (4n+2) electrons). Furan, Pyrrole, Thiophene, Indole, Benzofuran, Carbazole, Quinoline, Isoquinoline, Imidazole, Oxazole, Pyrazole, and Pyridazine are some examples of aromatic heterocyclic compounds.
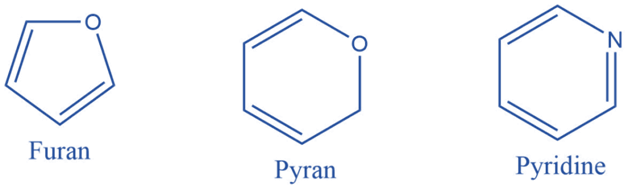
Uses of Hydrocarbons
- Hydrocarbons are commonly employed as fuels. Examples include LPG (Liquefied Petroleum Gas) and CNG (Liquefied Natural Gas).
- They are employed in the production of polymers such as polyethylene and polystyrene.
- As a starting material, these organic compounds are used in the manufacture of pharmaceuticals and colors.
- Hydrocarbons are found in oil supplements, vaccinations, injections, and pills in medicine. By themselves, these structures aren’t very useful in medicine, however, reactions can be used to add beneficial functional groups, creating pharmaceutical drugs.
- They are used as lubricants and grease.
- Heterocyclic compounds are widely used in pharmaceuticals, agrochemicals, and veterinary medicines. Many heterocyclic chemicals are extremely beneficial and necessary for human survival. Hormones, alkaloids, antibiotics, vital amino acids, hemoglobin, vitamins, dyestuffs, and pigments all contain a heterocyclic structure.
- Halogenated hydrocarbons are those in which hydrogen atoms are substituted with fluorine, chlorine, bromine, or iodine. In medicine, halogenated hydrocarbons are used to manufacture anesthetics such as halothane, propellants for inhalers, and sedatives such as chloral hydrate.
- Freon and other halogenated hydrocarbons are used as refrigerants.
- It is used on farms to heat animal shelters and greenhouses, for drying crops, managing pests and weeds, and powering agricultural machinery and irrigation pumps.
- Hydrocarbons are also used in powering forklifts, electric welders, and other equipment in companies and industries.
Environmental and Health Effects of Hydrocarbons
- Hydrocarbons can injure the lungs directly by breathing or produce systemic intoxication through ingesting, inhalation, or skin absorption. Many hydrocarbons are also irritating to the eyes and skin.
- Injecting hydrocarbons into the skin, subcutaneous tissue, or muscle can result in liquefaction necrosis and a severe local inflammatory reaction.
- On the other hand, many aromatic and halogenated hydrocarbons, alcohols, ethers, ketones, and other substituted or complex hydrocarbons have the potential to produce acute systemic toxicity, including coma, convulsions, and cardiac arrhythmias.
- Hydrocarbon-emitted gases have been found to affect respiratory systems and affect the environment through climate change and the greenhouse effect.
- By discharging pollutants, the oil and gas extraction process also causes significant damage to the surface environment and nearby groundwater of the extraction site. Unexpected spills pose a significant threat to marine and aquatic life.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
- https://www.vedantu.com/chemistry/aliphatic-hydrocarbons.
- https://byjus.com/chemistry/aromatic-compounds/#:~:text=Aromatic%20compounds%20are%20broadly%20divided,ring)%20for%20example%2C%20furan..
- https://ncert.nic.in/textbook/pdf/kech206.pdf.
- https://www.mcmsnj.net/cms/lib07/nj01911694/centricity/domain/136/chap21.pdf.
