Hund proposed a rule for the distribution of electrons in a given set of orbitals. For a particular electron configuration, the term with the largest multiplicity falls lowest in energy, according to the Hund’s Rule of maximum multiplicity. This rule states that electron pairing in the p, d, and f orbitals cannot take place until each orbital of a certain subshell has one electron in it or is entirely occupied.
This rule is based on the idea that because electrons are negatively charged, they repel one another. When two electrons move in the same region of space (e.g., the 2px orbital), they repel each other considerably more strongly than when they move in separate parts of space (e.g., the 2px and 2py orbitals). As a result, it is more energetically advantageous for them to move into different orbitals.
Interesting Science Videos
What is Hund’s rule of maximum multiplicity?
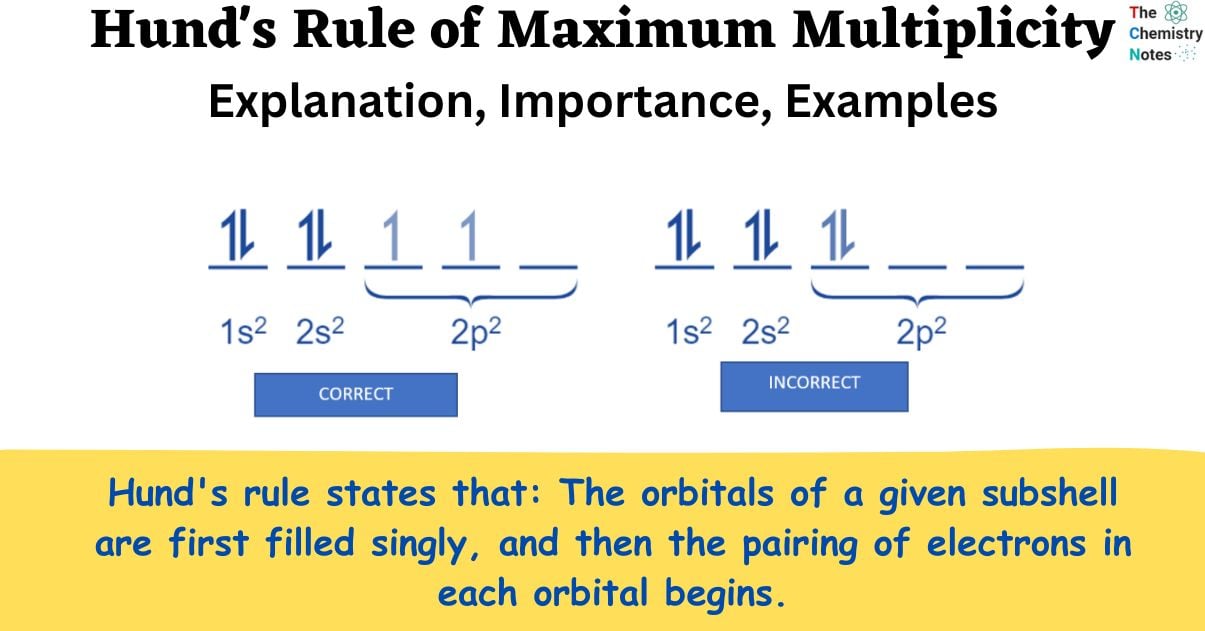
Hund’s rule states that: The orbitals of a given subshell are first filled singly, and then the pairing of electrons in each orbital begins.
In other words, electron pairing will not occur in any orbital until all accessible orbitals off of it contain at least one electron each. Hund’s rule is sometimes known as the “bus seat rule” since before pairing up, passengers select separate seats on a bus.
According to Hund’s Rule:
- Each orbital in the sub level is single-occupied before any orbital is double-occupied.
- All electrons in a single occupancy orbital have the same spin in order to maximize overall spin.
An electron will not pair with another electron in a half-filled orbital since it can fill all of its orbitals with equivalent energy. The atoms in the ground state have a large number of unpaired electrons. When two electrons collide, they behave similarly to two magnets. Before they must pair up, the electrons attempt to separate from one another.
In simple terms, the atomic state with the highest total spin quantum number is the lowest or most stable. Single electrons with the same value satisfy the rule since spin is either ½ or -½. Electrostatic repulsion between electrons is reduced by giving all of the single electrons in the orbitals the same spin. The classical example, while not fully correct, illustrates how electrons orbiting an atom all in the same direction collide less frequently than they would if some electrons traveled in one direction and some in the other. Basically, parallel spin is the most stable state, thus single electrons in subshells have it.
Explanation of Hund’s Rule with Examples
Before electrons pair up, the electrons enter an empty orbital. Due to their negative charge, electrons are attracted to one another. To lessen repulsion, the electrons do not share orbitals.
The spins of unpaired electrons in singly occupied orbitals are the same when the second rule is taken into account. The sub-level starting electrons spin determines what the spin of the subsequent electrons will be.
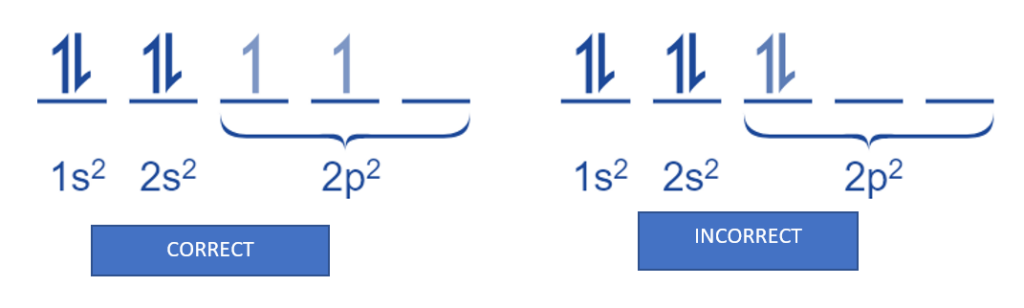
For example:
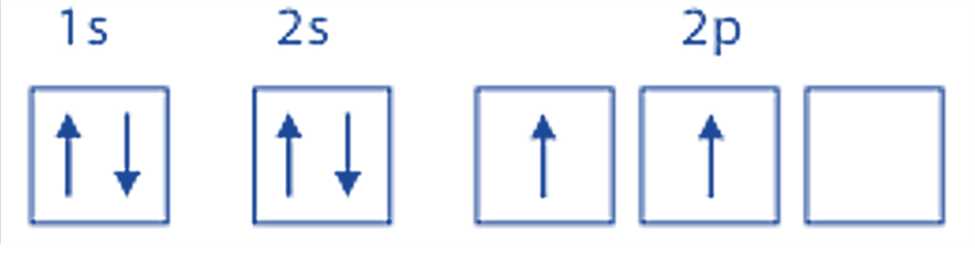
Carbon: The electron configuration of a carbon atom would be 1s2 2s2 2p2. According to Hund’s rule, the two 2p electrons will occupy different orbitals, whereas the two 2s electrons will occupy the same orbital.
Nitrogen: According to the nitrogen atom, electron configuration, nitrogen atom’s (Z=7) electron configuration is 1s2 2s2 2p3.
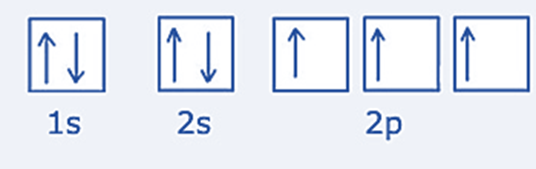
The subshells 1s and 2s are now filled. The 2p subshell is just half-filled. Accordingly, the three electrons in the 2p subshell are apart from one another and have the same spin, while the pairs of electrons in the 1s and 2s subshells are antiparallel and in pairs.
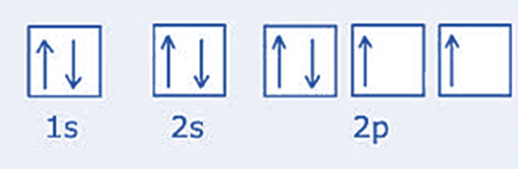
Oxygen: On the periodic table, oxygen is located after nitrogen (Z=8). It has a 1s2 2s2 2p4 electron arrangement. The 1s and 2s subshells are filled exactly as they are for nitrogen, but the 2p subshell has an extra electron. First, place one electron in each subshell. Make a pair with the extra electron by aligning it anticlockwise with the initial electron.
Importance of Hund’s Rule
- Hund’s rule is crucial because it demonstrates how electrons group into subshells.
- This distinguishes the valence electrons (the unpaired ones), which take part in chemical processes and are responsible for a large portion of an atom’s chemical characteristics.
- The stability of an atom, for instance, is reflected in its electron configuration. While an atom with no unpaired electrons is stable, one with one unpaired electron is highly reactive.
- An atom’s magnetic characteristics are also indicated by the valence shell. Atoms become paramagnetic and are drawn to magnetic fields if they contain unpaired electrons. A diamagnetic atom is one that is only weakly attracted to a magnetic field if all of its electrons are coupled.
Spin Multiplicity
The multiplicity of an energy level can be determined in spectroscopy and quantum chemistry using 2s+1, where s represents the total spin angular momentum. Singlets, doublets, triplets, quartets, and quintets are electron states with multiplicity 1, 2, 3, 4, and 5
Rule of Spin Multiplicity
According to the spin multiplicity rule, the lowest energy term for a given electron configuration is the one with the highest spin multiplicity value. This means that if two or more orbitals of equal energy are available, electrons will occupy them individually first before filling them in pairs.
Difference between Hund’s rule and Aufbau principle
The differences between the hund’s rule and Aufbau principle are mentioned below.
| Hund’s principle | Aufbau principle |
| Hund’s rule describes how the subshells of an atom are filled. | Aufbau’s principle describes how the orbitals of an atom are filled. |
| A single electron should occupy orbitals of the same energy initially, followed by the second electron. | It fills the electrons in the order of lowest to highest energy. |
| It describes how electrons fill subshells. | It describes the way in which electrons fill the orbitals. |
Frequently Asked Questions (FAQ)
Why is hund’s rule known as the rule of maximum multiplicity?
According to Hund’s rule, when filling up electrons in degenerate orbitals (orbitals with the same energy level), electrons fill up singly first, then pair up. It indicates that atomic orbitals have as many unpaired electrons as feasible. Maximum multiplicity is provided by a bigger number of unpaired electrons. As a result, Hund’s rule is known as the rule of maximum multiplicity.
What exactly is Hund’s rule?
Hund’s rule refers to an empirical rule for the arrangement of electrons in degenerate orbitals. According to Hund’s rule, “electrons are filled singly first in orbitals of the same subshell before pairing begins.”
What is the difference between Hund’s rule and Pauli exclusion principle?
Hund’s rule describes how electrons are filled in an atom’s subshells in pairs; no more than two electrons can occupy the same orbital, whereas Pauli’s rule states that no two electrons in a single atom may have the same quantum number or an identical set.
What are the applications of Hund’s rule?
It is commonly employed in spectroscopy to determine an atom’s precise electronic configuration, reactivity, and stability.
What type of configurations violate Hund’s rule?
If there is a double-occupied orbital before a singly filled orbital, Hund’s rule is violated.
Video on Hund’s Rule
References
- Cottingham, W. N.; Greenwood, D. A. (1986). “Chapter 5: Ground state properties of nuclei: the shell model”. An Introduction to Nuclear Physics. Cambridge University Press. ISBN 0-521-31960-9.
- Engel, T.; Reid, P. (2006). Physical Chemistry. Pearson Benjamin-Cummings. ISBN 080533842X.
- https://collegedunia.com/exams/hunds-rule-of-maximum-multiplicity-explanation-application-and-sample-questions-chemistry-articleid-776
- Goudsmit, S. A.; Richards, Paul I. (1964). “The Order of Electron Shells in Ionized Atoms”. Proc. Natl. Acad. Sci. 51 (4): 664–671. doi:10.1073/pnas.51.4.664
- Klechkovskii, V.M. (1962). “Justification of the Rule for Successive Filling of (n+l) Groups“. Journal of Experimental and Theoretical Physics. 14 (2): 334.
- https://byjus.com/chemistry/hunds-rule/
- https://school.careers360.com/chemistry/hunds-rule-topic-pge
- Miessler, G.L.; Tarr, D.A. (1999). Inorganic Chemistry (2nd ed.). Prentice-Hall. ISBN 0138418918.
- https://www.uppercareers.com/hunds-rule-of-maximum-multiplicity/
- https://general.chemistrysteps.com/hunds-rule/
- https://www.chemistrylearner.com/hunds-rule.html
- https://chemistrytalk.org/hunds-rule/
- https://sciencenotes.org/hunds-rule-definition-and-examples/