
A buffer solution is a water-based solvent-based solution made up of a weak acid and its conjugate base, or a base that is weak and its conjugate acid. They are resistant to pH fluctuations produced by dilution or the addition of trace quantities of acid or alkali.
The pH of buffer solutions varies minimally when just a tiny amount of strong acid or strong base is introduced. As a result, they’re employed to keep the pH stable.
In other words, a buffer solution (also known as a pH buffer or hydrogen ion buffer) is an aqueous solution comprised of a weak acid and its conjugate base, or vice versa. Buffer solutions are employed in a variety of chemical applications to maintain a nearly constant pH. Many life systems in nature employ buffering to control their pH.
The bicarbonate buffering system, for example, is used to maintain the pH of blood, and bicarbonate also works as a buffer in the ocean.
Preparation of Acidic Buffer
A strong base is combined with a weak acid and its salt to create an acid buffer, which has a pH of acidic.
Consider a weak acid (HA) and its salt (KA) in an acid buffer solution with a strong base (KOH). The equilibrium is as follows when the weak acid (HA) ionizes:
H2O + HA ⇋ H+ + A–
The acid dissociation constant is,
Ka = ([H+] [A–])/[HA]
RHS and LHS take negative logs:
-log Ka = -log [H+] – log ([A–]/[HA])
pKa = pH – log ([salt]/[acid])
pH = pKa + log ([salt]/[acid])
pH of acid buffer solution = pKa + ([salt]/[acid])
Preparation of Basic Buffer
Strong acid and a weak base and its salt are combined to create a basic buffer, which has a basic pH.
Consider a basic buffer solution that contains a strong acid, salt, and a weak base (B).
As a result, the basic buffer solution will be,
pOH = pKb + log ([salt]/[base])
pOH of a basic buffer solution = pKb + log ([salt]/[acid])
pH of a basic buffer solution = pKa – log ([salt]/[acid])
How to Prepare Buffer Solution with Specific pH
A pH-specific buffer solution can be made in a few different ways. When using the first approach, dissolve the buffer’s acid form in roughly 60% of the water needed to create the final solution volume in order to create a solution that contains acid and its conjugate base. A pH probe should then be used to determine the solution’s pH. A powerful base like NaOH can be used to raise the pH to the desired level. A strong acid like HCl can be used to change the pH of a buffer constructed from a base and its conjugate acid.
We must therefore select an appropriate acid/base pair with a specific concentration. A good acid should have a pKa that is as close as feasible to the ideal pH level.
The Henderson-Hasselbalch equation illustrates this.
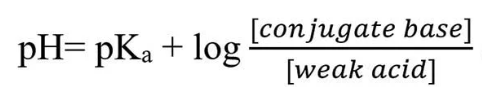
The Ka is the acid dissociation constant, and pKa is the negative log of Ka.
[Conjugate Base] is commonly written as [A–].
[Weak Acid] is written as [HA].
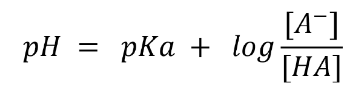
If we need a buffer with a pH of 3.75, an acid with a pKa close to 3.75 would be an excellent choice.
Difficulties of Preparing a Buffer Solution
- While the most basic buffer solution may consist of only an acid, a base, and water, lab workers are sometimes required to make many buffer solutions per day.
- The laboratory technician must first ensure that the correct buffer solution formula has been selected from a database of over 20 recipes. For buffer solution volumes of more than 1 liter, the quantities of all components must be recalculated and the new amounts logged. Any mistakes in the computations or recording of the new values could result in inaccurate pH values in the buffer solution. A manual data recording system is more prone to errors.
- When weighing in all of the different buffer solution components, it is critical to utilize the correct amount of each component. The true weight of each component must be recorded, and whether findings are recorded by hand or entered into a computer, care must be given to avoid transcribing errors.
- It may be necessary to utilize two distinct balances depending on how much of each buffer solution component is required and the minimum weight of the balance. This adds complexity to the operation and affects efficiency.
- It is critical to check the pH level of the buffer solution. However, pH readings may be erroneous if the pH meter is not properly calibrated and maintained. Using a buffer solution with an improper pH can have a significant impact on subsequent analyses and product quality.
- To minimize confusion, the final buffer solution must be appropriately labeled with all information. The expiry date is critical to ensuring that the buffer solution is still functional when employed. All data pertaining to the preparation of the buffer solution must be logged and securely maintained for future reference and accountability.
Video on How to Prepare Buffer Solution
References
- https://www.geeksforgeeks.org/buffer-solution/
- https://www.coursehero.com/study-guides/introchem/preparing-a-buffer-solution-with-a-specific-ph/
- https://unacademy.com/content/jee/ study-material/chemistry/preparation-of-buffer-solution/
- https://byjus.com/jee/buffersolutions/

