A homologous series is a group of chemical compounds that differ from one another by the methylene ( CH2) group. The most basic examples of homologous series are the first four hydrocarbons i.e., methane, ethane, propane, and butane, with chemical formulae CH4, CH3CH3, CH3CH2CH3. The CH2 group distinguishes ethane’s chemical formula from that of methane. Likewise, the formula of propane differs from the ethane formula by the CH2 group. This demonstrates that the homologous sequence develops sequentially. So, the alkanes form a series of compounds all with the general formula CnH2n+2.
When the –H atom in an alkane is replaced by some other group then they can form a different series of compounds. These atoms or groups attached are known as functional groups and the series formed are all homologous series. When the -H atom in an alkane is replaced by another group, a new sequence of compounds is formed. These associated atoms or groups are known as functional groups, and the developed series are all homologous series.
What is homologous series?
In organic chemistry, homologous series is a sequence of compounds with the same general formula and functional groups with similar properties which differs only in the carbon chain length. The members of the same homologous series are known as homologs. The members of this series can be branched or unbranched, or differ by the molecular formula of CH2 and molecular mass of 14u.
The compounds in the homologous series generally have a fixed set of functional groups that gives them similar physical as well as chemical properties. For example, the homologous series of alkanes is CH4, C2H6, C3H8, etc. They differ from each other by –CH2 unit.
Interesting Science Videos
Characteristics of Homologous Series
The following are some of the characteristics of the homologous series:
- Each member of the homologous series differs by a –CH2 unit.
- The functional group of the members of the homologous series is the same.
- The general formula for all the members of a homologous series is the same.
- The physical properties of the members of a homologous series may vary gradually with the increment of the molar mass. In general, as the molar mass increases, melting point, boiling point, and density increase.
- They have similar chemical properties. Under normal conditions, alkanes, for example, are fairly unreactive. They burn in the air, producing carbon(IV) oxide and water, and undergo substitution reactions with other compounds such as halogens.
- All members are typically prepared using the same general procedures, such as the action of hot soda lime on the suitable sodium salt of an acid.
Importance of homologous series
- Homologous series is a property of carbon compounds in which the carbon and hydrogen atoms in hydrocarbons vary by a single parameter.
- Homologous series aid in the identification of the structure of each consecutive member of the series and the properties of those members can also be anticipated by their series.
- It helps in predicting the properties of the series’ members who have yet to be prepared.
- Understanding homologous series allows for an organized investigation of the members.
- If the attributes of the first member are known, the nature of any member of the family can be determined.
- Even members of the series who are unprepared can benefit from being predicted in terms of their traits.
- Knowledge of homologous series is beneficial for the systematic study of organic chemistry because it reduces learning time.
Effects of Alkyl and Functional Groups
Alkyl group
Many homologous series can be considered as being produced from alkanes by substituting one or more hydrogen atoms with other elements or groups. The alkyl group is the univalent group produced from an alkane by the removal of a hydrogen atom. Thus, the compound generated via substitution is composed of the alkyl group and the substituent group.
All groups generated from alkanes by the removal of a hydrogen atom are referred to as alkyl groups. The general formula for alkyl groups is CnH2n+1. They are named after the parent alkanes by substituting the ending -ane with -yl. The physical properties of a chemical are affected by the alkyl group.
Functional group
A functional group is an atom or group of atoms that replaces hydrogen in an organic compound and is responsible for its distinctive chemical properties. Because of the durability of the carbon-hydrogen bonds, the alkyl group of a molecule is chemically quite inert. The substituent group mostly determines the chemical reactivity of an alkyl molecule. The hydroxyl group, -OH, the halogen groups -x ( X= F, Cl, Br, I), the amino group, -NH2, the carboxyl group, -COOH, the nitro group -NO2 and the double covalently connected carbon atoms, C=C are examples of functional groups. Each functional group has its own set of properties. When two or more functional groups coexist in a molecule, the properties of one are frequently altered or impacted by the presence of the others. Thus, the chemical characteristics of a homologous series are determined by the existence of the functional group or groups.
Summary
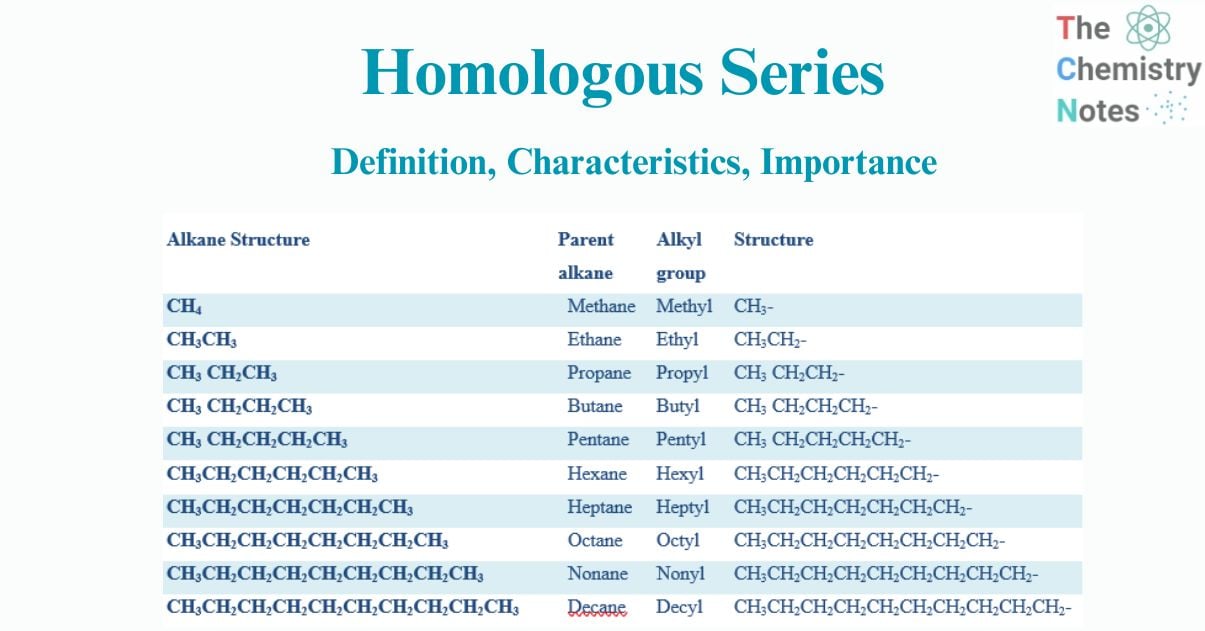
| S.N | Alkane Structure | Parent alkane | Alkyl group | Structure | For functional Group -OH |
| 1. | CH4 | Methane | Methyl | CH3– | CH3-OH |
| 2. | CH3CH3 | Ethane | Ethyl | CH3CH2– | CH3CH2– OH |
| 3. | CH3 CH2CH3 | Propane | Propyl | CH3 CH2CH2– | CH3 CH2CH2– OH |
| 4. | CH3 CH2CH2CH3 | Butane | Butyl | CH3 CH2CH2CH2– | CH3 CH2CH2CH2-OH |
| 5. | CH3 CH2CH2CH2CH3 | Pentane | Pentyl | CH3 CH2CH2CH2CH2– | CH3 CH2CH2CH2CH2– OH |
| 6. | CH3CH2CH2CH2CH2CH3 | Hexane | Hexyl | CH3CH2CH2CH2CH2CH2– | CH3CH2CH2CH2CH2CH2– OH |
| 7. | CH3CH2CH2CH2CH2CH2CH3 | Heptane | Heptyl | CH3CH2CH2CH2CH2CH2CH2– | CH3CH2CH2CH2CH2CH2CH2– OH |
| 8. | CH3CH2CH2CH2CH2CH2CH2CH3 | Octane | Octyl | CH3CH2CH2CH2CH2CH2CH2CH2– | CH3CH2CH2CH2CH2CH2CH2CH2– OH |
| 9. | CH3CH2CH2CH2CH2CH2CH2CH2CH3 | Nonane | Nonyl | CH3CH2CH2CH2CH2CH2CH2CH2CH2– | CH3CH2CH2CH2CH2CH2CH2CH2– OH |
| 10. | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 | Decane | Decyl | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2– | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2– OH |
References
- March J. (1977). Advanced organic chemistry : reactions mechanisms and structure (2d ed.). McGraw-Hill.
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- https://byjus.com/chemistry/homologous-series/.
- https://www.savemyexams.co.uk/a-level/chemistry/edexcel/17/revision-notes/3-organic-chemistry/3-1-introduction-to-organic-chemistry/3-1-2-homologous-series–functional-groups/.
- https://flexbooks.ck12.org/cbook/cbse-chemistry-class-10/section/4.6/primary/lesson/homologous-series-functional-groups-and-nomenclature/
