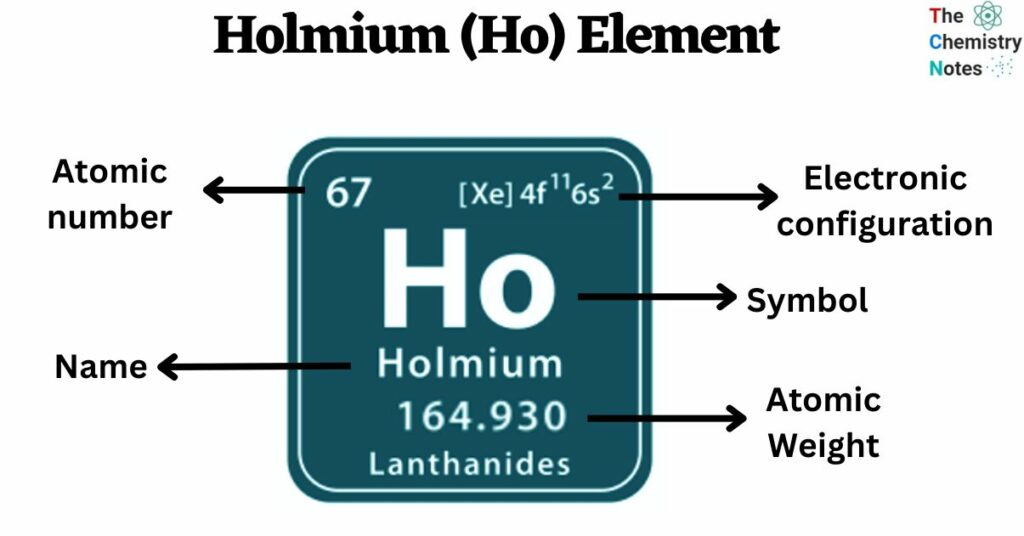
Holmium is a chemical element with an atomic number of 67 and is represented by the symbol ‘Ho’ in the periodic table. It is soft and silvery-white in appearance and classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. Like other rare earth elements, holmium is not naturally occurring in its pure form. It is found combined with other minerals elements, including gadolinite, monazite, and other rare-earth minerals.
A mineral with a dominant composition of holmium has not yet been discovered. Holmium constitutes approximately 1.4 parts per million of the Earth’s crust in terms of mass. As a result, it ranks as the 56th most prevalent element in the Earth’s crust.
Like other elements in the lanthanide series, holmium frequently occurs in the +3 oxidation state. It forms compounds like holmium (III) fluoride (HoF3) and holmium (III) chloride (HoCl3). In solution, holmium exists as Ho3+ ions coordinated with nine water molecules. Holmium is capable of undergoing dissolution when exposed to acidic environments. Nevertheless, holmium has been observed to exist in oxidation states of +2, +1, and 0.
Interesting Science Videos
History of Holmium
- In the year 1878, two chemists from Switzerland, Marc Delafontaine, and Jacques-Louis Soret, made an observation of spectroscopic lines that had not been documented before. The discovery of element ‘X, which is now known as holmium, was officially announced.
- In 1879, a Swedish researcher named Per Teodor Cleve made a significant discovery while investigating erbium oxide. He specifically identified two substances that were previously unknown. One of the substances had a brown color, while the other had a green color. The substance he initially referred to as holmia was later identified as holmium oxide.
- By using fractional precipitation, French chemist Paul Lecoq de Boisbaudran was able to isolate holmium oxide in 1886.
- In the year 1911, Otto Holmberg achieved the successful isolation of pure metal.
- The nomenclature of the chemical element holmium can be traced back to its etymological origin in the Greek term “holmia,” which is intrinsically linked to the geographical location of Stockholm.
Occurrence of Holmium
- Holmium comprises approximately 1.4 parts per million (ppm) of the Earth’s crust by mass. Consequently, it is positioned as the 56th most abundant element in the Earth’s crust.
- Holmium is commonly found in the +3 oxidation state. Holmium exhibits the ability to form chemical compounds such as holmium (III) fluoride (HoF3) and holmium (III) chloride (HoCl3).
- Holmium does not exist in its pure form in nature. It is commonly observed in association with various mineral constituents, such as gadolinite, monazite, and other minerals that belong to the rare-earth category.
- The commercial-scale extraction of this metal entails the application of ion exchange techniques, specifically targeting monazite sand as the primary source. The extraction of the metal is accomplished via a reduction mechanism, whereby the anhydrous fluoride of the metal undergoes a chemical reaction with calcium metal.
- Holmium exhibits a diverse range of isotopes, totaling 30 in number, each possessing distinct half-lives. These isotopes span across the mass numbers 141 to 172, contributing to the comprehensive understanding of Holmium’s nuclear properties. Holmium in its natural state is composed solely of its stable isotope, 165Ho.
Isotopes of Holmium
Holmium consists of a single naturally occurring isotope: 165Ho.
Naturally Occurring Stable Isotope
| Isotope | Natural Abundance (% atoms) |
|---|---|
| 165Ho | 100 |
Elemental Properties of Holmium
| Electronic Configuration | [Xe] 4f11 6s2 |
| Atomic Number | 67 |
| Atomic Weight | 164.9 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Lanthanides, 6, f-block |
| Density | 8.8 g/cm3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 29, 8, 2 |
| Electrons | 67 |
| Protons | 67 |
| Neutrons in the most abundant isotope | 98 |
Physical Properties of Holmium
- Holmium has an atomic number of 67 and is a silvery-white rare earth metal. It has a melting point of 1461 °C (2662 °F) and a boiling point of 2600 °C (4712 °F).
- Holmium has a solid phase density of 8.79 g/cm3 and a liquid or molten phase density of 8.34 g/cm3.
- It is malleable which means it can be easily beaten into thin sheets without any cleavage.
- It is ductile which means it is possible to draw thin wires from it without breaking.
- Holmium exhibits ferromagnetic properties when subjected to temperatures below 19K (-254.15°C), while its magnetic behavior transitions to a paramagnetic state under ambient conditions.
- Holmium possesses the most elevated magnetic moment when compared to all other elements that occur naturally.
- During periods of illumination, holmium oxide exhibits a discernible yellowish hue. When exposed to trichromatic light, the color of the object manifests as a shade of orange-red.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1734 K (1461 °C, 2662 °F) |
| Boiling point | 2873 K (2600 °C, 4712 °F) |
| Density | 8.79 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.23 (Pauling Scale) |
Chemical Properties of Holmium
- Similar to numerous lanthanides, holmium exhibits a sluggish reactivity when exposed to cold water, while displaying a comparatively accelerated reaction rate when subjected to hot water.
- Under normal circumstances, holmium demonstrates a significant level of stability when subjected to a dry atmospheric environment. Nevertheless, upon exposure to humid air, a chemical transformation occurs, giving rise to the creation of a yellow-hued oxide, thereby initiating the process of tarnishment.
- Holmium, an elemental metal, exhibits reactivity with all halogens, resulting in the formation of their respective trihalides. The sole trihalide exhibiting a pink hue is trifluoride, while the remaining trihalides manifest a yellow coloration.
- Similar to other elements in the lanthanide series, holmium typically exhibits a characteristic oxidation state of +3 in its compound formations.
Chemical Reaction of Holmium
- The Reaction of Holmium With Water
The element holmium exhibits a sluggish reaction when exposed to cold water, resulting in the formation of holmium hydroxide (Ho(OH)3) and the liberation of hydrogen gas (H2).
2 Ho (s) + 6 H2O (g) → 2 Ho(OH)3 (aq) + 3 H2 (g)
- The Reaction of Holmium With Air
At ambient conditions, the reaction between holmium and oxygen is characterized by a sluggish rate, while it exhibits a pronounced tendency to combust, ultimately resulting in the formation of holmium(III) oxide, denoted as Ho2O3.
4 Ho (s) + 3 O2 → (g) 2 Ho2O3 (s)
- The Reaction of Holmium With Halogens
The element holmium exhibits a propensity to engage in chemical reactions with various halogens, resulting in the formation of holmium (III) halides.
The chemical reaction between holmium metal and fluorine gas (F2) results in the formation of holmium (III) fluoride, denoted as HoF3.
2 Ho (s) + 3 F2 (g) → 2 HoF3 (s) [Pink]
The chemical reaction between holmium metal and chlorine gas (Cl2) results in the formation of holmium (III) chloride, denoted as HoCl3.
2 Ho (s) + 3 Cl2 (g) → 2 HoCl3 (s) [Yellow]
The chemical reaction between holmium metal and bromine (Br2) results in the formation of holmium (III) bromide, denoted as HoBr3.
2 Ho (s) + 3 Br2 (g) → 2 HoBr3 (s) [Yellow]
The chemical reaction between holmium metal and iodine represented as I2, results in the formation of holmium (III) iodide, denoted as HoI3.
2 Ho(s) + 3 I2 (g) → 2 HoI3 (s) [Yellow]
- The Reaction of Holmium With Acid
In addition to the release of hydrogen gas (H2), the process of dissolving holmium metal in diluted sulfuric acid results in the creation of solutions containing the yellow-aquated Ho(III) ion. It is highly probable that the predominant form of Ho3+ (aq) exists as the complex ion [Ho(OH2)9]3+.
2 Ho (s) + 3 H2SO4 (aq) → 2 Ho3+ (aq) + 3 SO42- (aq) + 3 H2 (g)
Uses of Holmium
Regardless of the radioactive nature and the limited evidence regarding its toxicity, the utilization of holmium in both industrial and research settings is justified due to its numerous applications. The manifold applications of Holmium in various facets of daily existence are enumerated herewith.
Used In Magnets
Holmium serves as a flux concentrator for numerous high magnetic fields and is employed as an alloy in the fabrication and manufacturing of magnets. Holmium, by virtue of its distinctive magnetic properties, serves as a pivotal element in the fabrication of the most potent artificially produced magnetic fields.
Used In Lasers
The utilization of holmium-doped yttrium lithium fluoride (YLF) and yttrium iron garnet (YIG) is prevalent in the field of solid-state devices. In the production of Cubic Zirconia and Glass, the utilization of Holmia, also known as holmium oxide, is favored due to its ability to impart a natural yellow and red coloration.
Used For Calibration
In order to achieve accurate calibration, optical spectrophotometers exhibit a preference for utilizing Holmium as a standard reference material. The utilization of Holmium-166m1, an enduring radioactive isotope, serves the purpose of calibrating gamma-ray spectrometers.
Used For Treatments
In the context of non-invasive medical procedures, the utilization of the element Holmium (Ho) is observed in solid-state specialized lasers, which find application in various domains including cancer therapy, fiber-optic technologies, dental surgeries, and the fragmentation of kidney stones. Holmium is primarily utilized in therapeutic interventions for the ocular condition known as glaucoma as well as for the rectification of unsuccessful or erroneous glaucoma surgical procedures. Holmium lasers are a useful tool for mitigating the atypical range of intraocular pressure in human eyes.
Quantum Computers
IBM has successfully devised a methodology for the storage of a singular unit of information, specifically one bit, utilizing a solitary holmium atom that is strategically positioned upon a substrate composed of magnesium oxide. Holmium exhibits promising prospects for utilization in the realm of quantum computing.
Health Effects of Holmium
- Holmium does not possess any discernible biological function and is regarded as one of the scarcest elements found within the human body.
- The observation has been made that holmium possesses the ability to enhance metabolic activity, despite its seemingly low acute toxicity rating.
Environmental Effects of Holmium
- Holmium does not present any discernible environmental hazard to the flora and fauna.
References
- https://www.rsc.org/periodic-table/element/67/holmium
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Mary Elvira Weeks, The Discovery of the Elements XVI., Journal of Chemical Education., October 1932, p1761,1762; 1a. p1767.
- https://pubchem.ncbi.nlm.nih.gov/element/Holmium
- Robert E. Krebs, The history and use of our earth’s chemical elements: a reference guide., JGreenwood Publishing Group, 2006, p296, p300.
- Tables of Physical & Chemical Constants, Kaye & Laby Online, 16th edition, 1995. Version 1.0 (2005), accessed December 2014.
- https://byjus.com/chemistry/holmium/
- J. S. Coursey, D. J. Schwab, J. J. Tsai, and R. A. Dragoset, Atomic Weights and Isotopic Compositions (version 4.1), 2015, National Institute of Standards and Technology, Gaithersburg, MD, accessed November 2016.
- https://www.chemicool.com/elements/holmium.html

