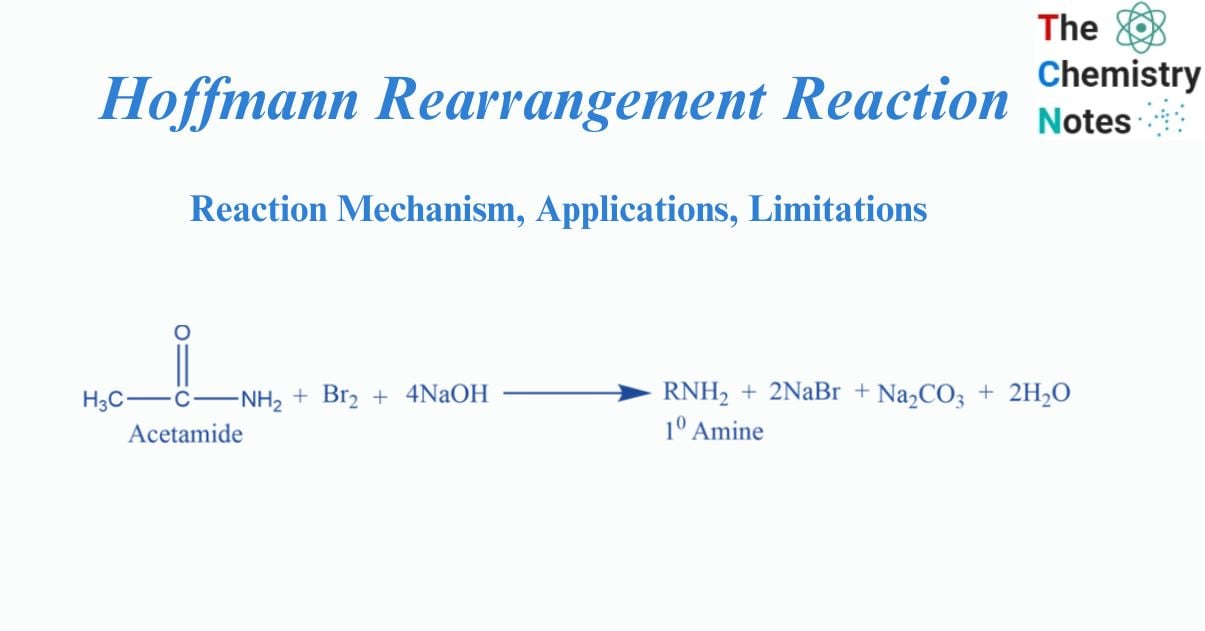
Hoffmann bromamide reaction involves the conversion of an amide into a primary amine with one carbon less, by the reaction of amide with bromine in an aqueous NaOH.
August Wilhelm Von Hoffmann described Hoffmann’s bromamide degradation process. It is also known as Hoffmann Rearrangement Reaction. This reaction produces primary amines. An alkyl or aryl group moves from the amide’s carbonyl carbon atom to the nitrogen atom during this degradation reaction.
Interesting Science Videos
What is Hoffmann bromamide reaction ?
Warming of the amide with bromine and the concentrated aqueous solution of NaOH solution form primary amine. It is a good method for the laboratory preparation of primary amine. This reaction is also called Hofmann’s degradation reaction. The product contains one carbon less than the original amide.

Mechanism of Hoffmann bromamide reaction
Step 1: The primary amide and sodium hydroxide interact in this step. The sodium hydroxide ion attacks the primary amide, resulting in the production of water and the development of a negative charge in the primary amide ion. This occurs as a result of primary amide dehydrogenation.
Step 2: In this step, primary amide ions and the bromine molecules react with each other. During the reaction, the bromine-bromine link break, one bromine atom acquires a partially positive charge, while the other bromine atom develops a partially negative charge. The bromine attacks the nitrogen to form bromamide.
Step 3: The base now attacks the N-Bromamide again, causing deprotonation and the production of water, and the formation of a bromamide anion.
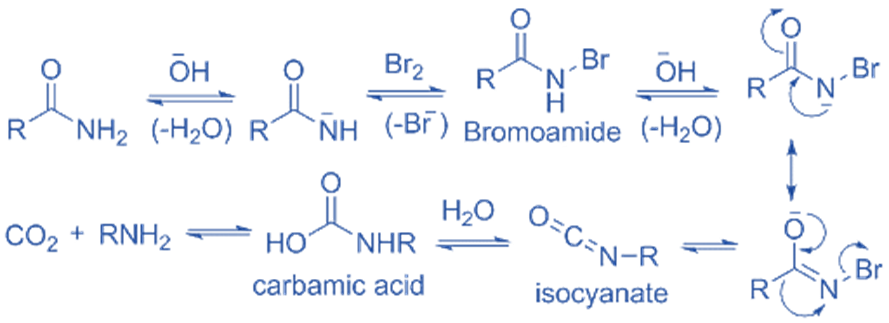
Step 4 – This bromamide anion undergoes rearrangement such that any R- group that was previously attached to the carbonyl carbon now connects with nitrogen. Simultaneously, the bromide anion generated departs the complex. As a result, an isocyanate is formed.
Stage 5: A nucleophilic addition reaction occurs when water is added to isocyanate, resulting in the formation of carbamic acid.
Step 6: The carbamic acid now releases carbon dioxide, resulting in a negatively charged nitrogen that is bound to one hydrogen and the ethyl group. When this is protonated by water, the required primary amine is generated.
Application of Hoffmann bromamide reaction
- Nitriles are formed when higher amides (those with more than eight carbons) are reduced to amines.
- The important reagent hydrazine is produced by urea.
- It’s employed in the synthesis of aliphatic amines.
- It is used in the synthesis of anthranilic acid. Anthranilic acid is used in a variety of industrial processes, such as the production of saccharin, an artificial sweetener, azo dyes, and perfumes.
- It is used in the synthesis of fundamental aromatic compounds.
- The Hoffmann Bromamide degradation reaction can be utilized to produce 3-Aminopyridine from nicotinic acid.
- It is employed in the synthesis of phthalimide and aniline.
Limitation of Hoffmann bromamide reaction
Only primary amides undergo Hoffmann Bromamide reaction. This reaction does not occur in secondary or tertiary amides.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- Arun Bahl, and B.S. Bahl (2006). A textbook of organic chemistry Chemistry. New delhi: S. CHAND.
