Heterocyclic compounds contain at least one heteroatom (atom other than carbon) in the cyclic ring structure. The three most frequent heteroatoms are nitrogen (N), oxygen (O), and sulfur (S). Heterocyclic compounds are commonly found in plants and animal products, and they are a key element of over half of all known natural organic compounds. Some examples of natural heterocyclic compounds include alkaloids, natural colors, medicines, proteins, enzymes, and so on.
Saturated heterocyclic molecules act similarly to acyclic derivatives but with different steric properties. The usual amines and ethers in this category are piperidine and tetrahydrofuran. However, because of their unstrained nature, unsaturated heterocyclic compounds with 5- and 6-member rings have received a great deal of attention. Pyridine, Thiophene, Pyrrole, Furan, and it’s benzo fused derivatives are examples of unstrained unsaturated heterocyclic compounds. Many of these heterocyclic aromatic chemicals are components of key biological molecules, such as DNA and RNA bases and pharmaceuticals.
Interesting Science Videos
Nomenclature of heterocyclic compounds
The Hantzch-Widmann nomenclature system is the most important system approved by IUPAC for the nomenclature of heterocyclic compounds. The nature, position, ring size, number, and type of heteroatoms present in each heterocyclic compound are all specified by this naming system.
Name: Prefix + Stem + Suffix
The following are significant considerations for the systematic nomenclature of heterocyclic compounds.
1. In this nomenclature, the nomenclature of heterocyclic compounds is assigned by combining the ‘prefix’ (which indicates the presence of a heteroatom) with the stem (which indicates the ring size as well as the saturation and unsaturation in the ring) and the suffixes. When the prefix is followed by a vowel, the final ‘a’ is dropped.
2. the nomenclature of heterocyclic compounds begins with the heteroatom. If a heterocyclic compound contains more than two separate heteroatoms, the prefixes are stated in the order.
3. When two or more hetero atoms of the same kind are present in a heterocyclic compound, they are denoted by di-, tri-, and so on.
4. The position of the saturated atom is denoted numerically by the prefix ‘H-‘ as part of the ring system’s name. It should be noted that where there is a choice of numbering, the indicated place is given the lowest possible number.
5. The stem indicates the size of a monocyclic ring (three to ten membered rings).
Prefix for heteroatom in preferential order
| S.N | Heteroatom | Symbol | Prefix |
| 1. | Oxygen | O | Oxa |
| 2. | Sulfur | S | Thia |
| 3. | Selenium | Se | Selena |
| 4. | Nitrogen | N | Aza |
| 5. | Phosphorous | P | Phospha |
| 6. | Arsenic | As | Arsa |
| 7. | Antimony | Sb | Stiba |
Prefix for Heteroatoms in preferential order.
| S.N | Ring size | Unsaturated Ring | Saturated Ring |
| 1. | 3 | iren | Irane |
| 2. | 4 | ete | Etane |
| 3. | 5 | Ole | Olane |
| 4. | 6 | ine | Inane |
| 5. | 7 | epine | Epane |
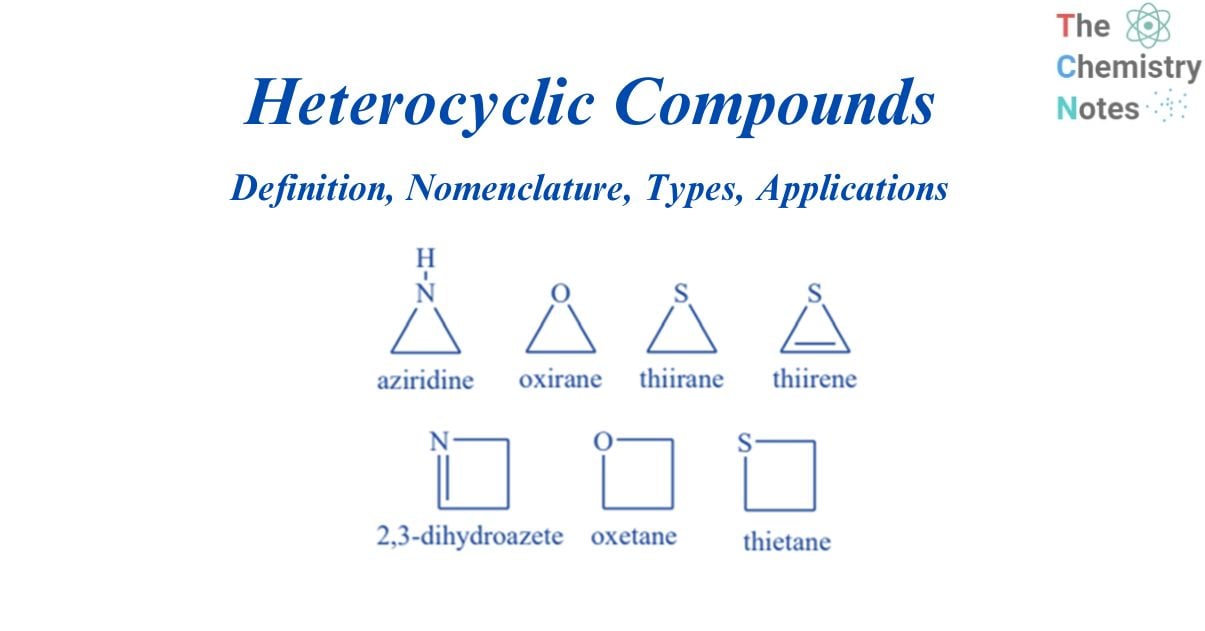
Some examples of heterocyclic compounds
Types of heterocyclic compounds
Based on the structure the compound is classified into five different types they are:
- Three-Membered Heterocyclic Compounds
- Four-Membered Heterocyclic Compounds
- Five-Membered Heterocyclic Compounds
- Six-Membered Heterocyclic Compounds
- Condensed or Fused Heterocyclic Compounds
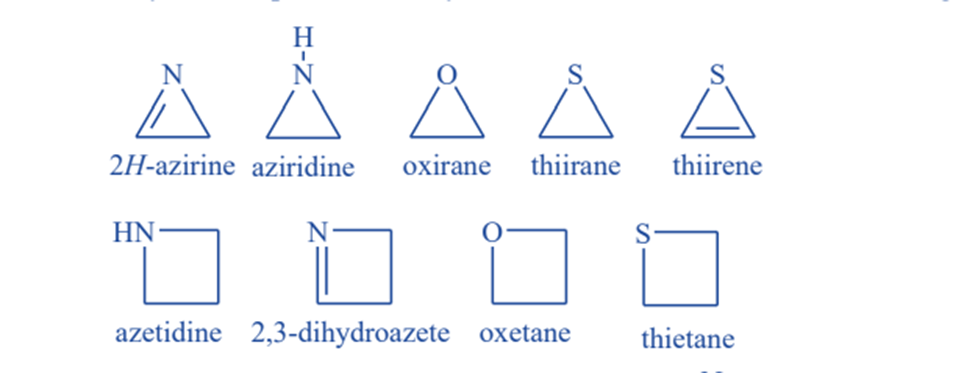
Three-membered ring compounds
Aziridine, oxirane (or ethylene oxide), and thiirane are three-membered ring heterocycles containing single atoms of nitrogen, oxygen, and sulfur, respectively.
The Gabriel ring closure: The aziridine was first produced in 1888 by heating ß-bromomethylamine in the presence of KOH.

The most prevalent three-membered heterocycles are oxiranes (epoxides). Epoxides are easily synthesized by reacting alkenes with peracids, and they typically have high stereospecificity.
One of the most common ways of epoxidation is the addition of an oxygen atom to an alkene with peroxycarboxylic acids.
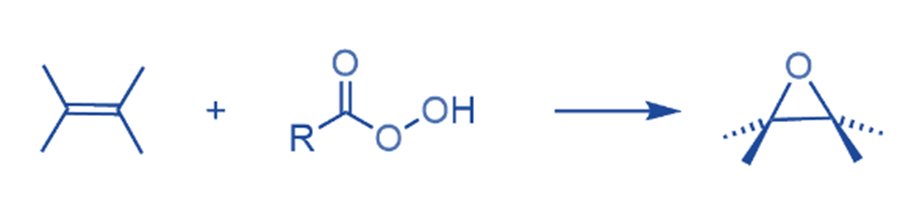
Epoxides are more reactive than unstrained ethers due to the high-angle strain of the three-membered ring. The most general reaction class is addition reactions that proceed via electrophilic or nucleophilic ring opening.
The oxirane ring can also be opened via hydrolysis, which is the breaking of a bond followed by the addition of water components (H and OH). This reaction produces ethylene glycol (HOCH2CH2OH), which is used as an antifreeze in cooling and heating systems, brake fluid, and as a solvent in the paint and plastics industries.
Alkenes react with electrophilic sulfur reagents to generate α-halo-β-sulfur intermediates, which can subsequently be induced to cyclize to thiiranes.
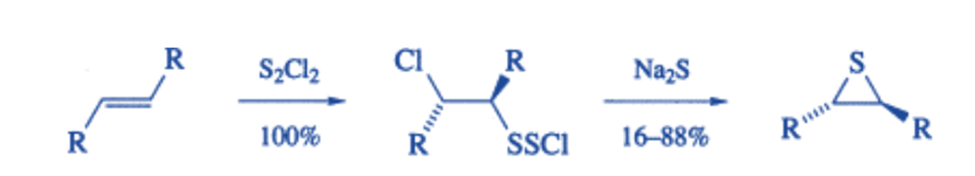
Thiirane-ringed molecules are more bactericidal than oxirane-ringed molecules, and some thiirane derivatives have been used as tuberculostats (drugs that inhibit the growth of tuberculosis-causing bacteria), whereas thiirane 1 oxides are insecticides, molluscicides, or herbicides.
Four-membered ring compounds
These heterocyclic compounds have four atoms that can be either saturated or unsaturated. Azetidine, oxetane, and thietane are four-membered rings that each contain one nitrogen, oxygen, or sulfur atom.
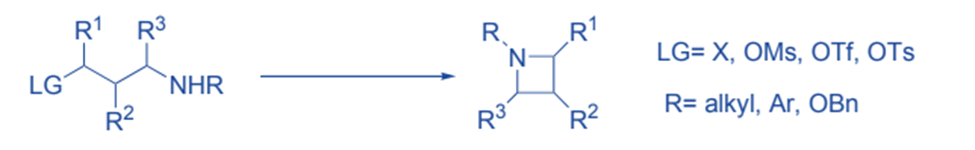
Azetidines can be prepared by cyclization by nucleophilic substitution of amine nucleophiles.
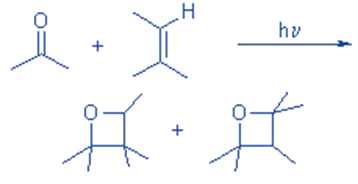
( 2+ 2) cycloaddition of alkene to the carbonyl compounds produces oxetane.
1,3 dibromo propane reacts with Na2S to produce thietane.
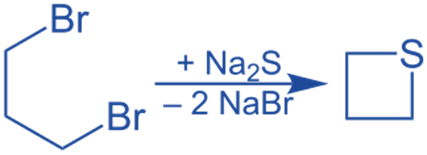
Five-membered rings
There are five atoms in these heterocyclic rings. This family’s parent aromatic chemicals are pyrrole, furan, and thiophene.
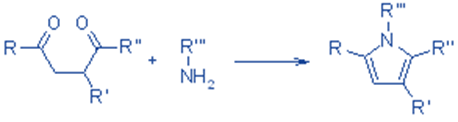
A pyrrole is formed via the condensation of a 1,4-dicarbonyl molecule with an excess of a primary amine or ammonia.
Pyrrole rings can be found in the amino acids proline and hydroxyproline, which are found in many proteins and are especially abundant in collagen, the structural protein of bones, tendons, and skin.
The acid-catalyzed cyclization of 1,4-dicarbonyl compounds produces furan. It is known as the Paal-Knorr synthesis. Many sugars exist in molecular forms known as furanoses, which have the tetrahydrofuran ring structure. Maleic anhydride and phthalic anhydride are industrially important furan derivatives that are components of resins and polymers.
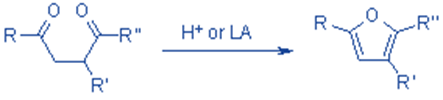
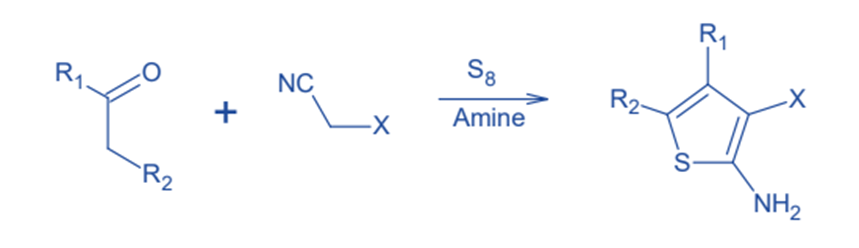
Gewald developed this approach in 1966. Gewald synthesis is the most common method for producing 2- aminothiophenes. It is made up of the base-catalyzed condensation of a ketone with a CH2 group and a ß-ketonitrile to create an olefin, followed by cyclization with elemental sulfur.
Six-membered ring compounds
These heterocyclic compounds are created by substituting one of the carbon atoms with a hetero atom containing a single pair of electrons. Pyridine is a common example of six-membered heterocyclic compounds. Pyridine, as well as picolines, lutidines, and collidines, can be found in coal tar and bone oil. Pyridine derivatives are extremely important in biology. For example, nicotinic acid is more widely known as niacin, a B-complex vitamin.
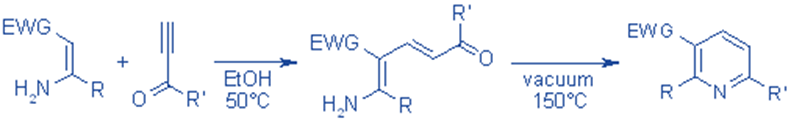
The Bohlmann-Rahtz Pyridine Synthesis produces substituted pyridines in two stages. Condensation of enamines with ethynyl ketones produces an amino diene intermediate, which undergoes cyclodehydration after heat-induced E/Z isomerization to produce 2,3,6-trisubstituted pyridine.
Applications of heterocyclic compounds
- Agrochemicals and pharmaceuticals both use heterocyclic compounds.
- Heterocyclic compounds are utilized as starting materials in organic chemical synthesis.
- In corrosion inhibitors, sanitizers, anti-ordinates, and developers, heterocyclic chemicals are utilized.
- Pesticides, dyes, and plastics all contain heterocyclic compounds.
- They serve as industrial solvents, intermediates in the manufacture of other compounds, and corrosion inhibitors. Epoxides are also utilized to make polymers, adhesives, and sealants.
- Pyrrole and its derivatives are frequently employed as intermediates in the manufacture of pharmaceuticals, pharmaceuticals, agrochemicals, dyes, photographic chemicals, fragrances, and other organic compounds.
- They are utilized as polymerization catalysts, preservatives, and solvents for resin and terpenes.
- They serve as the gold standard for chromatographic analysis.
- They are essential raw ingredients in the chemical industry.
- They are utilized in dental care products as an antimicrobial.
- They are employed as a solvent suited for dehalogenation.
- They are employed as a disinfectant.
- They are utilized as a ligand in coordination chemistry.
