Water has an extraordinarily high boiling point temperature, close to 100°C. The heat of vaporization is the term for the amount of energy needed to convert one gram of liquid water into water vapor due to the network of hydrogen bonds that exist between water molecules. The heat of vaporization of water is 40.65 kJ/mol. This change in water requires a significant quantity of heat energy (586 calories). This happens on the water’s surface. As liquid water heats up, hydrogen bonding makes it difficult to separate the water molecules from one another, allowing it to enter its gaseous phase (steam).
What is the Heat of Vaporization of Water?
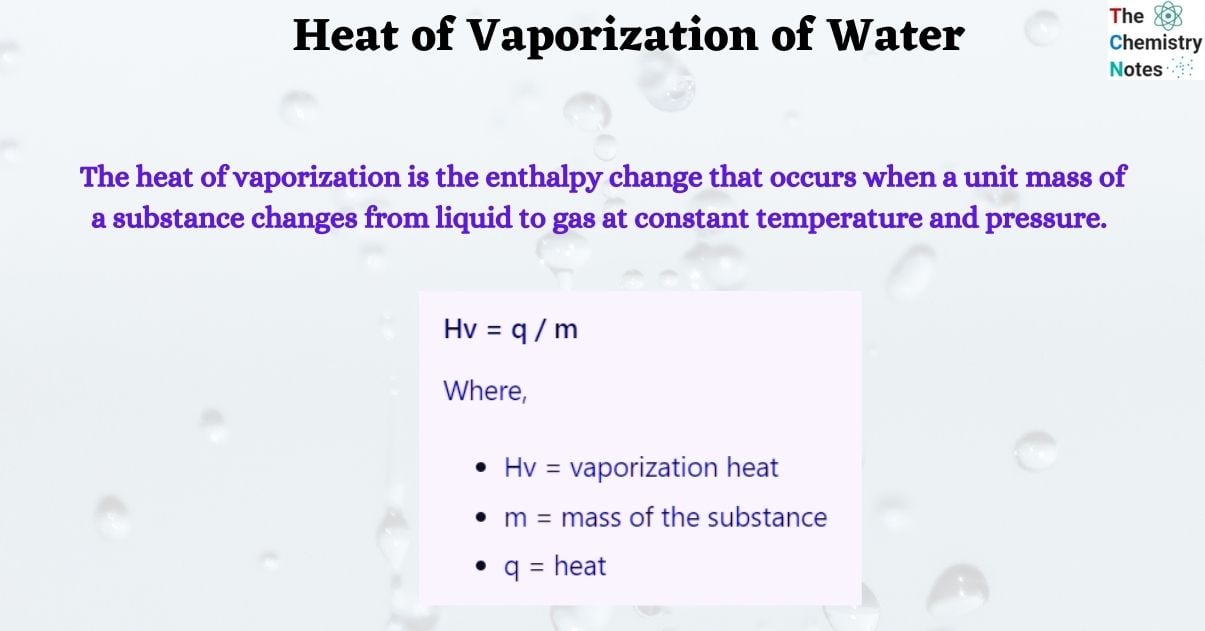
The heat of vaporization of water is the enthalpy change that occurs when a unit mass of a substance (water) changes from liquid to gas at constant temperature and pressure. It is also known as the enthalpy of vaporization or the latent heat of vaporization.
Water evaporation provides a cooling effect, whereas condensation provides a heating effect. Thus, the heat of vaporization is the amount of heat that must be absorbed in order to create vapors of a certain amount of liquid at a constant temperature. When comparing vapor solutions and liquid states, steam has a higher kinetic energy than liquid. An increase in energy causes liquid particles to evaporate during the vaporization process by overcoming intermolecular interactions.
A precise amount of heat is required to cause physical changes and switch the states of a substance. The heat of vaporization or enthalpy of vaporization is the amount of energy or heat used per unit mass during vaporization.
Heat of Vaporization Formula
The Heat of vaporization formula can be expressed using entropy and enthalpy of vaporization and their relationship.
Hv = q / m
Where,
- Hv = vaporization heat
- m = mass of the substance
- q = heat
Why Heat of Vaporization is Always Positive?
Enthalpy is constantly added to a system in order to evaporate a liquid, hence the heat of vaporization is always positive. More kinetic energy increases the likelihood that the molecules will separate from the liquid and turn into a gas. It is possible to think of the required increase in internal energy as the energy required to dismantle the intermolecular connections in the liquid. Less energy is required to break bonds between atoms that are stronger than they are.
The amount of energy required is temperature-dependent and depends on the pressure at which the transition occurs. The less energy required, the hotter the liquid already is. More energy is required at greater pressures. The heat of vaporization vanishes at a critical temperature (Tr=1). After this temperature, the substance is no longer identifiable as a liquid or a vapor. Instead, it is referred to as a supercritical fluid.
Because vapor particles can flow more freely than liquid particles in a solution including both the liquid and gaseous states, the kinetic energy of the vapor is greater than that of the liquid. Heat and pressure are produced by the greater mobility of gas particles in comparison to liquid particles.
Heat of Vaporization Example
The heat of vaporization of water is 2146 Joules/gram, or 510 calories/gram. What amount of energy (in Joules) is necessary to turn 50 grams of water into steam? How much energy is there in a calorie?
Solution
m = 50 grams
ΔHv = 2146 J/gram
Q = m · ΔHv
Q = (50 g) · (2146 J/g)
Q = 107300 J = 107.30 kJ
In calories:
ΔHv = 510 cal/g
Q = m · ΔHv
Q = (50 g) · (510 cal/g)
Q = 25500 cal = 25.5 kcal
To turn 50 kilos of liquid water into gaseous water or steam, 107300 Joules or 25500 calories of heat are required.
Molar Heat of Vaporization
The molar heat of vaporization or molar enthalpy of vaporization is the change in enthalpy that occurs when one mole of a substance transitions from liquid to gas. Water has a molar heat of vaporization of 40.67 kJ/mol.
H2O (l, water) → H2O (g, water vapor) ΔHvap= 40.67 kJ/mol
Because vaporization requires energy, the molar enthalpy of vaporization has a positive sign. This shows that the system absorbs energy to get the molecules into the gas state.
The formula for the Molar Enthalpy of Vaporization
The following is the mathematical formula for calculating the molar enthalpy of vaporization:
q = n⋅ΔHvap
q = the amount of absorbed heat
n = the number of moles
ΔHvap is the molar enthalpy change of vaporization
This equation is rearranged to give:
ΔHvap = q/n
Video on Heat of Vaporization of Water
References
- https://sciencenotes.org/heat-vaporization-example-problem/
- https://www.khanacademy.org/science/ap-biology/chemistry-of-life/structure-of-water-and-hydrogen-bonding/a/specific-heat-heat-of-vaporization-and-freezing-of-water
- https://biologydictionary.net/heat-of-vaporization/
- https://www.thoughtco.com/definition-of-molar-enthalpy-of-vaporization-605361
- Helmenstine, Todd. “Heat of Vaporization Example Problem.” ThoughtCo, Feb. 16, 2021, thoughtco.com/heat-of-vaporization-example-problem-609499.
- https://collegedunia.com/exams/heat-of-vaporization-formula-physics-articleid-5152
- https://byjus.com/heat-of-vaporization-formula/

