Halogenation of alkene involves the addition of a halogen such as bromine or chlorine to an alkene’s double bond. Because of the polarizability of their covalent bond, halogens can act as electrophiles. Halogen addition is stereospecific, producing vicinal dihalides with anti-addition.
Halogen dissociates into an ion with opposite charges during halogenation and attacks the double bond to generate dihalide.
All of the halogens are not readily engaged in the halogenation of alkenes. Br and Cl are popular halogens employed in this type of reaction. Because of the size of its atom, I is too slow for this reaction, but F is far too strong and explosive. In this reaction, inert solvents (for example, CCl4) can be employed.
The halogens Br2 and Cl2 react with an alkene’s double bond to form vicinal dihalides, which are compounds with the halogens on nearby carbons (vicinus, Latin for “adjacent”). These are also known as 1,2-dihalide.
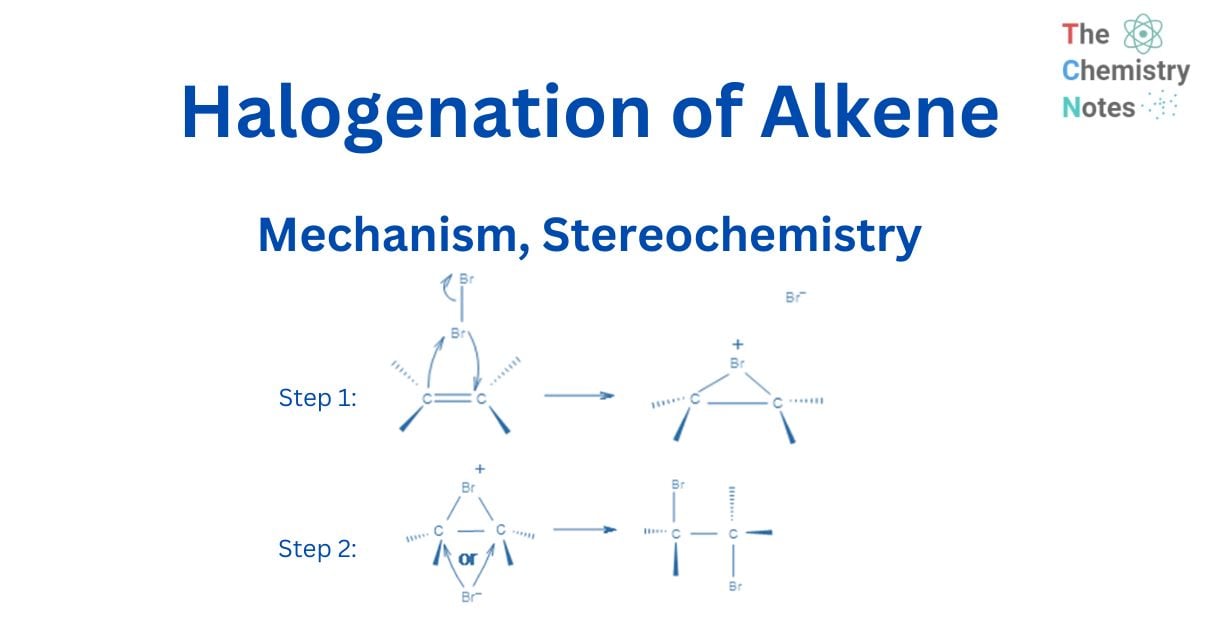
As a halogen molecule, such as Br2, approaches an alkene’s double bond, electrons in the double bond repel electrons in the bromine molecule, creating polarisation of the halogen bond. This results in the formation of a dipolar moment in the halogen molecule bond. When a heterolytic breakdown occurs, one of the halogens gains a positive charge and responds as an electrophile. The reaction of the addition is stereoselective rather than regioselective. The mechanism of the reaction can explain the stereochemistry of this addition.
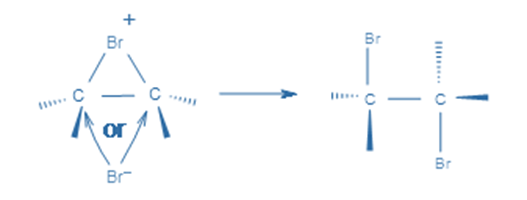
In the first stage, an electrophilic halogen with a positive charge approaches the double carbon bond, binds with two carbon atoms, and forms a cyclic ion with a halogen as an intermediary step. In the second stage, like in the SN2 reaction, halogen with a negative charge attacks any of the two carbons in the cyclic ion from the back side of the cycle. As a result, the product’s stereochemistry is vicinal dihalides via anti-addition.
Interesting Science Videos
Mechanism and stereochemistry of Halogenation of alkene
The bond’s p electrons attack the Br2 to form a new bond with it, and the other bromine leaves with the electron pair. This, however, does not result in a carbocation since the bonded bromine’s electron cloud is very close to the other sp2 carbon and makes a new connection with it. As a result, instead of the common carbocation, a cyclic bromonium ion intermediate is generated in the addition reaction.
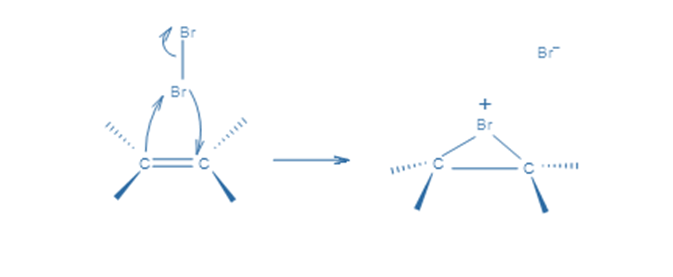
This cyclic intermediate is a three-membered ring that is unstable due to the high strain and highly susceptible to nucleophilic attacks, as observed in the oxymercuration reaction. Bromide anion attacks any carbon of the bridging bromonium ion from the back side of the cycle in the second phase. The cyclic intermediate opens, and two halogens are in the anti position.
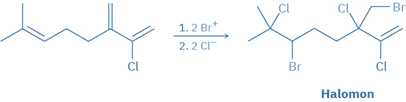
Alkene halogenation processes occur in nature in the same way that they do in the laboratory, but are mostly limited to marine creatures living in halide-rich environments.
These biological halogenation reactions are carried out by haloperoxidases, which employ H2O2 to oxidize Br- or Cl– ions to the biological equivalents Br+ or Cl+. Electrophilic addition to a substrate molecule’s double bond produces a bromonium or chloronium ion intermediate, which is subsequently completed by a reaction with another halide ion. Halomon, an anticancer pentahalide isolated from the red alga, is assumed to be formed via a two-step addition of BrCl via the corresponding bromonium ions.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
- https://byjus.com/chemistry/alkene/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/08%3A_Alkenes-Reactions_and_Synthesis/8.02%3A_Halogenation_of_Alkenes-_Addition_of_X
- https://www.chemistrysteps.com/halogenation-of-alkenes-and-halohydrin-formation/
