The Haber’s process, commonly known as the Haber-Bosch process, is the primary industrial approach for ammonia synthesis. One of the most effective and productive industrial processes for producing ammonia is the Haber process. By combining airborne nitrogen and hydrogen, primarily from natural gas (methane), the Haber Process creates ammonia. Ammonia is produced exothermically and the process is reversible.
N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g) + 22 Kcal
One of the most important industrial chemical processes ever devised is the Haber Process. The technology made ammonia fertilizer widely available, contributing to a global population boom as agricultural outputs grew substantially in a short period of time.
Interesting Science Videos
History of Haber’s Process
Ammonia is synthesized on an industrial scale using three main processes: Haber’s Process, Cyanamide’s Process, and Serpeck’s Process. The Haber process is known for being a cost-effective method of synthesis.
- Dr. Fritz Haber, a German scientist and Nobel Laureate, made a fundamental contribution to the science of chemistry by discovering an efficient process for generating ammonia. This method involves directly mixing hydrogen and nitrogen gas under particular circumstances, resulting in the inexpensive manufacture of ammonia.
- Dr. Haber’s seminal work in this sector has had a long-lasting influence on the discipline of chemistry. He was born in the German city of Breslau, which is today known as Wroclaw, Poland. He earned his schooling at Berlin’s Technische Hochschule. After finishing his chemistry studies, he was appointed Professor of Physical Chemistry at the University of Berlin in 1911.
- In 1918, he was awarded the Nobel Prize in Chemistry for his pioneering work in the invention of synthetic ammonia, fertilizers, and explosives. He is commonly regarded as “The Father of Chemical Warfare” for his contributions to the invention and deployment of chlorine and deadly gases during World War I. Karl Bosch, a German chemist, commercialized the process in the 1930s.
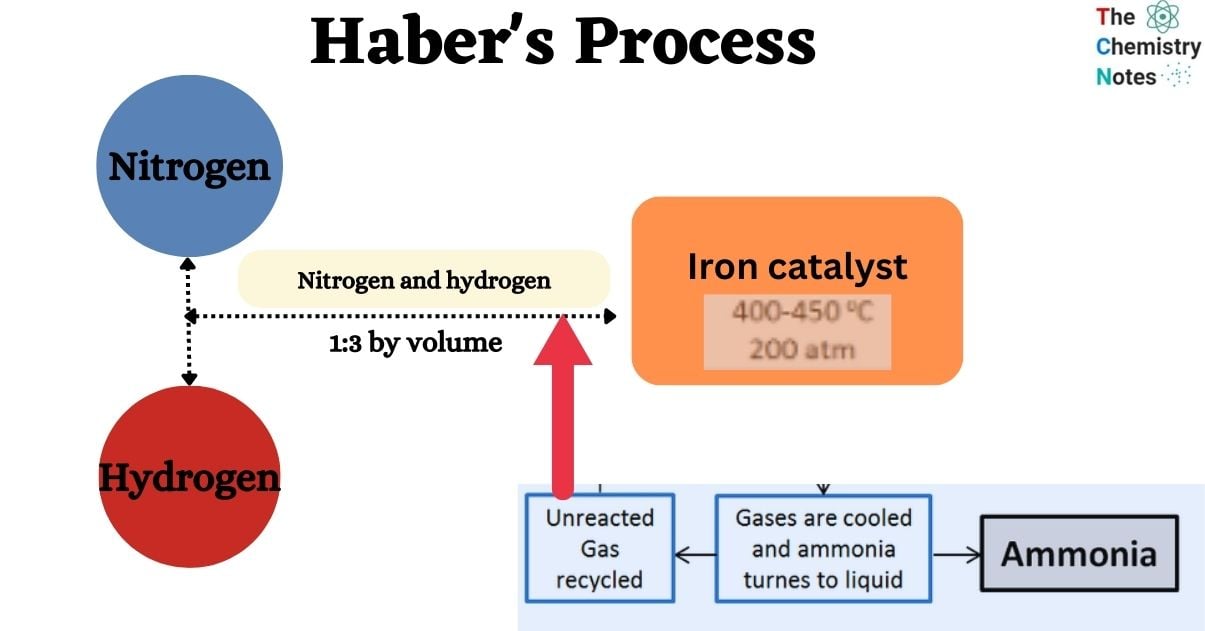
Manufacturing Ammonia Using Haber’s Process
Raw Materials
The manufacturing of ammonia necessitates the use of many materials. The use of these key materials is required for the synthesis of ammonia.
- Nitrogen
- Hydrogen
- The utilization of Al2O3 and K2O as promoters for iron and
- Zirconium dioxide (ZrO2) as catalyst promoter.
Principle
Nitrogen is an inert gas that does not react or mix with hydrogen under typical conditions.
- Heating a mixture of dry nitrogen and hydrogen can result in the production of ammonia.
- When measuring by volume, the ideal volume ratio for this mixture is 1:3.
- The reaction occurs in the presence of a catalyst, often promoted iron with trace concentrations of metal oxides such as Al2O3, K2O, and ZrO.
- The reaction takes place at temperatures ranging from 450°C to 500°C and pressures ranging from 200 to 900 atm.
N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g) + 22 Kcal
1 vol. 3 vol. 2 vol.Conditions For High Yield of Ammonia
Given the aforementioned synthetic reaction’s reversible nature, exothermic activity, and inclination to occur with a decrease in volume, it is expected that Le Chatelier’s principle would imply the following beneficial circumstances:
Low Temperature
- According to Le-Chatelier’s principle, lower temperatures are beneficial for ammonia synthesis based on the exothermic nature of the equilibrium reaction.
- In the realm of industrial ammonia production, the feasibility of relying on the reaction between Nitrogen gas (N2) and Hydrogen gas (H2) at temperatures below 450 °C is considered unviable due to its inherent sluggishness.
- Consequently, a temperature condition between roughly 450 °C and 500 °C is adopted.
High Pressure
- The equilibrium reaction involving the gaseous compounds hydrogen (H2) and nitrogen (N2) exhibits a phenomenon wherein the volume of the resulting product experiences a reduction.
- Pressure increases, ranging from 200 to 900 atmospheres, stimulate the favored production of ammonia.
High Concentration
- The reaction proceeds in the forward direction as the volume diminishes.
- It is advised to use an excess of one or both of the reactants, especially hydrogen (H2) or nitrogen (N2), to maximize the synthesis of ammonia (NH3).
Catalyst
- The incorporation of a catalyst serves to accelerate the rate of the chemical reaction kinetics.
- It is highly recommended to employ finely dispersed iron, accompanied by aluminum oxide (Al2O3), potassium oxide (K2O), and zirconium dioxide (ZrO2) as a promoter, in order to enhance the overall performance.
Purity of Hydrogen and Nitrogen
- In order to maintain optimal catalytic activity, it is crucial to ensure that the nitrogen and hydrogen gases used are of high purity.
- If these gases are contaminated, the catalyst can become poisoned, leading to a decrease in its effectiveness.
Details of the Process
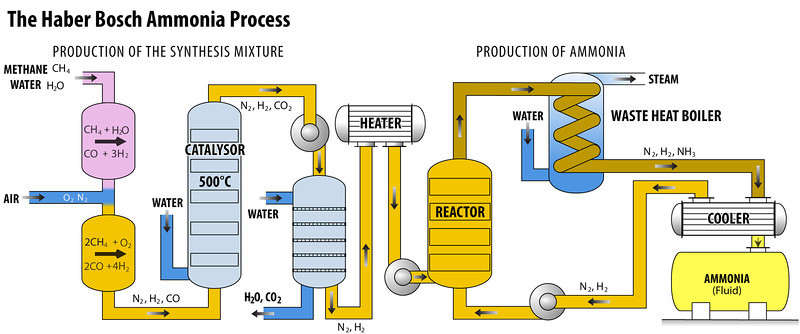
Production of Hydrogen and Nitrogen
- There are two ways to obtain hydrogen gas: electrolysis of Water or the creation of water gas (CO + H2).
- In the first procedure, water is electrolyzed to separate hydrogen and oxygen.
- The second technique combines carbon monoxide (CO) and hydrogen (H2) to form water gas, which is then fractionally liquefied to eliminate the carbon monoxide.
- Nitrogen is obtained through a process called fractional distillation of liquid air.
Compressor
- The gaseous elements nitrogen and hydrogen, in a volumetric ratio of 1:3, undergo compression within the purifying unit.
- This compression process is facilitated by a compressor pump, which operates at pressures ranging from 200 to 900 atmospheres.
Purifying Unit
- After that, the compressed gas is sent into a soda-lime tower, which is made of sodium hydroxide and calcium oxide, also known as soda-lime.
- This substance may absorb carbon dioxide (CO2) and moisture from its environment.
2 NaOH + CO2 → Na2CO3 + H2OCaO + H2O → Ca(OH)2Catalyst Chamber
- A vertical, cylindrical steel tank with thick walls is intended to handle high gaseous pressure.
- The material is made of finely split iron that has been enhanced with Al2O3, K2O, and ZrO.
- Carl Bosch designed the machinery and catalysts for Haber’s process, which resulted in the well-known Haber-Bosch ammonia synthesis.
- To begin the process, the chamber is electrically heated to roughly 500 °C. The process is exothermic, which implies that heat is released as a byproduct.
- This heat generated by the reaction helps sustain and continue the reaction.
- At the specified temperature and pressure, roughly 15% of the reactant gases are converted to ammonia.
Condenser
- The gas produced by the catalyst chamber contains ammonia as well as unreacted hydrogen and nitrogen. Following processing, the mixture is passed through a condenser.
- Ammonia condenses in this condenser and is collected in a receiver.
- Unreacted hydrogen (H2) and nitrogen (N2), on the other hand, stay gaseous and do not condense.
- Ammonia that has been condensed is referred to as liquor ammonia.
Recirculation
- A recirculation pump is required to circulate the uncondensed gas, which is predominantly composed of unreacted nitrogen and hydrogen, through the catalytic converter.
- This technique allows the gas to be reprocessed, resulting in the generation of more ammonia.
Advantages of Haber’s Process
- The economic benefits of the Haber process are a substantial advantage. This method of manufacturing ammonia is a cost-effective way of producing fertilizer that may be used to boost crop yields. This improves global food security by allowing farmers to produce more with less land, resulting in higher productivity.
- Another advantage is the process’s versatility, which allows producers to make additional valuable compounds such as nitric acid, urea, and ammonium nitrate. These compounds are widely used in a variety of sectors, including medicines, explosives production, and refrigeration.
Disadvantages of Haber’s Process
- The Haber’s process is one of the most energy-intensive processes. The interaction between nitrogen and hydrogen necessitates temperatures of up to 450°C and pressures exceeding 200 atmospheres, resulting in significant amounts of energy required for heating and cooling equipment.
- Another disadvantage is that greenhouse gases such as carbon dioxide are produced during the manufacturing process, which contributes to global warming. Alternative catalysts or operating at lower pressure conditions might be used to alleviate these difficulties.
Frequently Asked Questions (FAQ)
Why is Haber’s process important?
The Haber’s process is still used today because it produces ammonia, which is essential for fertilizer and many other applications. Every year, the Haber cycle generates around 500 million tons (453 billion kg) of fertilizer. This fertilizer contributes to the feeding of around 40% of the world’s population.
Why is iron employed as a catalyst in the Haber’s process?
Iron is utilized as a low-cost catalyst in the Haber cycle. It enables for a decent yield in a reasonable amount of time. Mention three reaction conditions that are controlled in industrial processes.
Why is the Haber’s process a hazardous reaction?
Generating the necessary high pressure demands a significant amount of energy, which is quite expensive. The dangers of high pressures are real. Due to the hydrogen gas being used, using high pressures may be extremely risky since they might result in explosions.
Haber’s process emits a significant quantity of CO2. 1.87 tons of CO2 are released into the atmosphere along with every ton of ammonia generated, which contributes 1.2% of the world’s total CO2 emissions and lowers average global temperatures.
Video on Haber’s Process
References
- https://www.studysmarter.co.uk/explanations/chemistry/the-earths-atmosphere/haber-process
- https://www.vedantu.com/chemistry/haber-process
- https://cen.acs.org/environment/green-chemistry/Industrial-ammonia-production-emits-CO2/97/i24
- https://oxsci.org/the-haber-process-a-simple-discovery-that-changed-the-world/
- https://www.studypug.com/chemistry-help/the-haber-process

