Graphite is one of the most frequent allotropes of carbon. It is also the most stable allotrope of carbon and is thus utilized in electrochemistry as the reference state for defining the heat of carbon compound synthesis. It occurs as hexagonal crystals, flexible sheets, scales, or masses. Under high pressure and temperatures, it converts into a diamond. It may have an earthy, grainy, or compressed texture.
The metamorphism of carbonaceous sediments and the interaction of carbon compounds with hydrothermal fluids produce graphite. Graphite has a wide range of characteristics and applications. It is the most stable form of carbon under normal conditions and occurs naturally.
Types Of Graphites
There are two types of graphite, which are discussed here:
Natural Graphites
Natural graphite comes in three varieties; all are made from naturally derived graphite. Amorphous graphite, flake graphite, and crystalline vein graphite are the three types, and each has unique features that make it well-suited for specific uses.
Flake Graphite: When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. The carbon source material can be organic or inorganic; however, most commercially available flake graphite is derived from organic deposits. Carbon concentrations fluctuate between 5% and 40%. Graphite flakes can be discovered within metamorphic rocks that include limestone, gneisses, and schist rock in the form of lamellae or scaly.
Amorphous Graphite: The least graphitic form of organic graphite is amorphous graphite. Amorphous graphite can be found in the beds of mesomorphic rocks such as coal, slate, or shale deposits as minute particles. Amorphous graphite, as a seam mineral, has a higher ash percentage than other types of graphite. Its graphite concentration is 25% to 85%, depending on geological context. While all natural graphite must be processed because of its low graphite content, amorphous graphite requires the most intensive processing.
Crystalline Vein Graphite: According to popular belief, crystalline vein graphite is generated from crude oil reserves that eventually turn into graphite with the passage of time, temperature, and pressure. Although the actual genesis of crystalline vein graphite is unknown, it is thought to be a naturally occurring pyrolytic. Although this type of natural graphite can also be found in the United Kingdom and the United States, it is only sourced and processed in Sri Lanka.
Synthetic Graphite
It’s made by a complicated process of baking petroleum coke at extremely high temperatures. Synthetic graphite can have a purity of more than 99% carbon and is utilized in manufactured products that require an incredibly pure material.
Electrographite, for example, is pure carbon produced in an electric furnace from coal tar pitch and calcined petroleum coke. Synthetic graphite is produced by heating calcined petroleum pitches to 2800 degrees Celsius. The electrical resistance and porosity of synthetic graphite is higher, but the density is lower. It is not suitable for refractory applications because of its high porosity.
Graphite Occurrence
It is created by the metamorphism of sedimentary carbonaceous material and the reduction of carbon compounds, which are components of igneous rocks. It occurs in metamorphic rocks as the reduction of sedimentary carbon compounds. It can also be found in magmatic rocks and meteorites. Quartz, calcite, mica, and tourmaline are minerals that are related to it.
Structure of Graphite
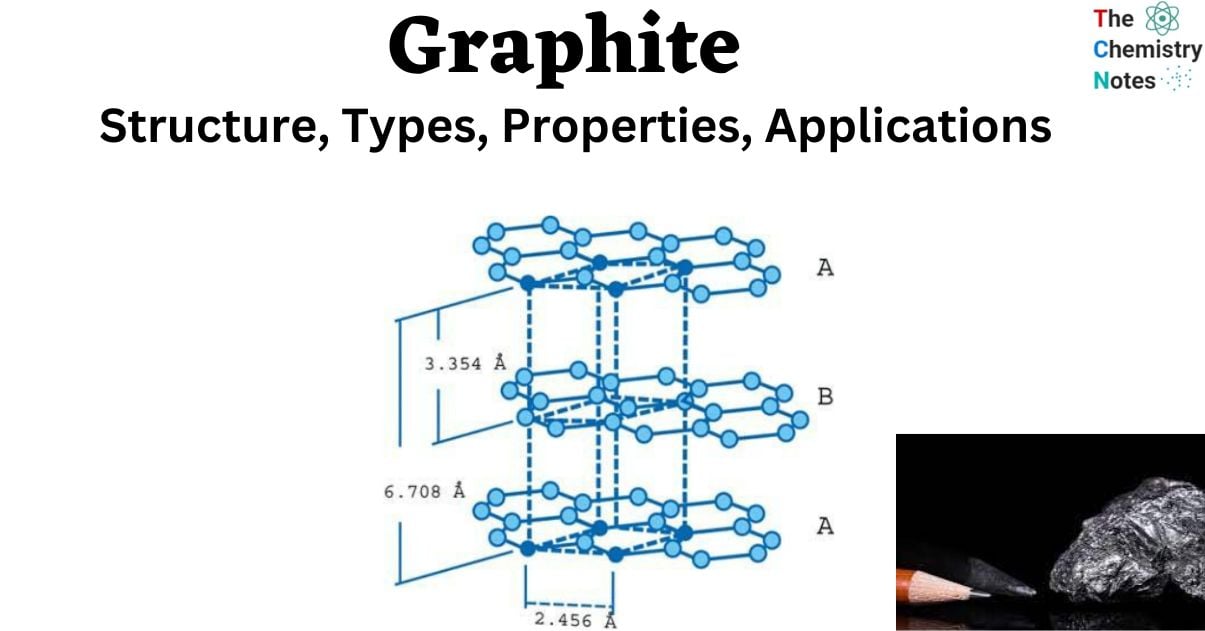
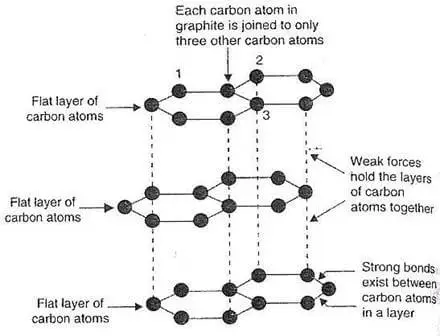
Graphite is made up of layers of carbon atoms organized in hexagonal rings with six members. These rings are joined together at the edges. Layers of fused rings can be represented mathematically as an infinite succession of fused benzene rings (without the hydrogen atoms).
[Image source: Engineeringchoice]
- The carbon atoms in these ring arrays are sp2-hybridized.
- Each carbon atom in the sp2 molecular orbital model is connected to three other species, three other carbon atoms in the case of graphite.
- The bond angle between adjacent carbon atoms in this bonding mode is 120 degrees.
- These “ring arrays” are made up of vast sheets of carbon atoms, and individual sheets are referred to as graphene layers. In a layer plane, the carbon-carbon bond length is 1.418.
- Graphene layers are layered parallel to the “C” crystallographic axis of the hexagonal 4-axial system in which graphite crystallizes.
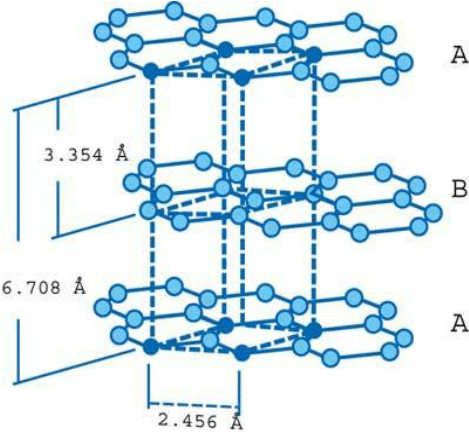
- Each layer’s carbon atoms are arranged in a honeycomb lattice with a bond length of 0.142 nm and a plane spacing of 0.335 nm. The spacing between the layers is 2.5 times the distance between each layer’s atoms.
- Carbon atoms in the first layer indexed are precisely aligned with carbon atoms in the third layer indexed. Similarly, the carbon atoms of the second graphene layer align with the atoms of the fourth layer. This is referred to as a “ABABAB” structure.
[Image source: https://www.researchgate.net]
Physical Properties Of Graphite
Electrical Conductivity: Graphite is an excellent electrical conductor due to its free delocalized electrons, which can freely travel throughout the sheets. It has higher electrical conductivity when compared to diamond.
Thermal Conductivity: Graphite is also a good thermal conductor because it has a very low coefficient of thermal expansion. However, diamond is a better thermal conductor due to its compact atoms.
High Melting Point: Graphite has an extremely high melting point of about 3500 °C.
Lubrication: The layers of graphite are bonded together by weak forces, which allow one layer to slide over another. As a result, the structure can be sliced along plane lines. As a result, black lead is pliable and slick.
Density: Because of the great distance among successive layers, its density is 2.26 g/cm3, which is much lower when compared to the density of a diamond.
Lustre: Graphite includes delocalized electrons that can freely migrate through multiple levels of the graphite lattice, reflecting any light that strikes them. The surface seems glossy or lustrous because the reflection is specular rather than dispersed.
Insoluble: Because the attraction between solvent molecules and carbon atoms is not strong enough to overcome the covalent connections between the carbon atoms in graphite, it is insoluble in organic solvents and water.
| Composition | Carbon |
| Color | Dark gray to black |
| Streak (color when crushed to a powder) | Black gray |
| Luster | Metallic to dull |
| Crystal structure | Hexagonal |
| Cleavage | Basal in direction 1,1 |
| Melting temperature | about 6420°F (3550°C) |
| Density | 2.26 g/cm3 |
| Hardness | 1 – 2 Mohs |
| Fracture | Conchoidal (smooth shell-like) |
| Name | Origin from the Ancient Greek graphein (to write). |
Chemical Properties Of Graphite
- Reaction With Oxygen
Carbon, in the form of graphite, burns to produce gaseous carbon (IV) oxide (carbon dioxide), CO2. Diamond is a type of carbon that, when heated to 600°C-800 °C, also burns in the air.
C (s) + O2 (g) → CO2 (g)
When the availability of air or oxygen is limited, incomplete combustion occurs, resulting in carbon monoxide, CO.
2 C (s) + O2 (g) → 2 CO (g)
This reaction is significant. Air is blown through hot coke in industry. Producer gas is a mixture of carbon monoxide, carbon dioxide, nitrogen, and trace amounts of hydrogen (H2), methane (CH4), and oxygen (O2).
- Reaction With Water
Under normal conditions, carbon in the form of graphite does not react with water. Under specific conditions, the described reaction occurs and produces water gas, which is a mixture of carbon monoxide and hydrogen gas.
C + H2O → CO + H2
- Reaction With Halogen
At high temperatures, graphite combines with fluorine, F2, to produce carbon tetrafluoride, CF4, as well as some C2F6 and C5F12.
C (s) + excess F2 (g) → CF4 (g) + C2F6 + C5F12
Other halogens do not appear to react with graphite.
- Reaction With Acids
Mellitic acid, C6(CO2H)6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid.
Applications Of Graphite
Chemical Industry
Graphite is used in numerous high-temperature applications in the chemical industry, such as the manufacturing of phosphorus and calcium carbide in arc furnaces. Graphite is utilized as an anode in aqueous electrolytic processes such as halogen (chlorine and fluorine) synthesis.
Refractory material
Graphite is a particularly valuable refractory material due to its excellent temperature stability in the absence of oxidizing chemicals. At 3600- 37000 degrees Celsius, the vapor pressure of graphite exceeds one atmosphere. High refractoriness is complemented by heat conductivity, resistance to thermal shock and slag, and chemical inertness. Graphite is used to make a variety of refractory materials, including metallurgical crucibles, retorts, muffles, saggars, pouring nozzles, and so on.
Turbo-jets
Because of its ability to generate mechanical strength at temperatures as high as 2400°C, graphite has recently been employed successfully in the manufacture of turbo-jet nozzles. Small vanes used in rockets are also made of dense graphite and must withstand temperatures of up to 2750°. Attempts have recently been undertaken to replace cobalt base alloy turbine rotor blades with graphite blades, which are lighter and can sustain higher temperatures.
Electrodes
Electrodes, heating elements, carbon brushes, electrical contacts, rheostats, plates, discs, rods, and electric plungers are all made from graphite.
Atomic Energy
In the generation of nuclear energy, very pure graphite is employed as a matrix enclosing uranium metal bars. The graphite matrix serves as a moderator, slowing down the rapid neutrons in the atomic pile to the level required for resonance capture by uranium atoms U238 and eventual transformation into plutonium Pu239•.
Pigments & paints
Flaky graphite’s water repellent characteristics, as well as its oily and chemically inert properties, make it an ideal material for the paint and pigment industries. Steel paint is frequently made from graphite black paint. Red lead and aluminum paints have been shown to survive longer when combined with graphite. Graphite paints are also used to keep wood in good condition.
Pencils
The pencil and crayon industry accounts for around 8% of global output. This task is best suited to the very fine soft variant.
Lubricants
The most important solid lubricant is graphite, which may be used with either water or oil. This feature results from the weak pressures between planes, allowing it to glide easily on basal cleavage. The presence of water, ammonia, and hydrocarbon coatings is also significant for graphite’s lubricating qualities.
Carbon Graphene Sheets
Single graphene sheets that have been rolled are 10 times lighter and 100 times stronger than steel. This type of rolled sheet is also known as graphene, and this graphite derivative is the world’s strongest recognized substance, having been used to construct super-strength, lightweight sporting equipment.
Its strong electrical conductivity, low light absorption, and chemical resistance make it a promising material for future applications such as medical implants, flexible electronic devices, and aircraft parts.
Frequently Asked Questions (FAQ)
What is the composition of graphite?
Graphite is a naturally occurring crystalline allotropic form of carbon. It can be made artificially by heating a sand-coke mixture in an electrical furnace at around 3300 K. Carbon atoms in graphite are sp2 hybridized. Covalent bonds connect the carbon atoms.
Why can graphite conduct electricity while diamond cannot?
The only nonmetal that conducts electricity is graphite. Because one carbon atom is bonded to three carbon atoms in graphite, and one carbon atom is free to generate electricity.
Where can you find graphite in nature?
Graphite is found in metamorphic rocks like marble, schist, and gneiss. Graphite is found in metamorphic rocks like marble, schist, and gneiss. It is the most stable allotropic form of carbon in terms of thermodynamics. It has a metallic shine and ranges in color from steel grey to black depending on the origin.
Why graphite is soft?
Graphite layers might slide over each other due to weak intermolecular interactions. Graphite has a soft feel as a result of this.
Is graphite a metal?
Graphite is a non-metal.
How does graphite act as a lubricant?
Carbon atoms are tightly attached and organized in sheets. Because the bonds that hold the sheets together are weak, graphite has a lower shear strength under friction force.
Video on Graphite
References
- https://www.engineeringchoice.com/what-is-the-structure-of-graphite/
- https://collegedunia.com/exams/graphite-structures-applications-properties-and-uses-science-articleid-6374
- https://www.azom.com/article.aspx?ArticleID=1630
- https://www.toppr.com/guides/chemistry/carbon-and-its-compounds/graphite/
- https://semesters.in/structure-applications-graphite/
- https://byjus.com/chemistry/graphite/
- Nightingale, R. E.. Graphite: Advantages, Limitations, And Applications. United States: N. p., 1966. Web. doi:10.2172/4524408.
- https://www.engineeringchoice.com/what-are-the-uses-of-graphite/
- A Note on Properties and Uses of Graphite: https://doi.org/10.1080/0371750X.1955.10877668
- https://asbury.com/resources/education/graphite-101/structural-description/

