Germanium is a metallic element with the atomic number 31 and is represented by the symbol ‘Ge’ in the periodic table. It is classified as a metalloid, which shows the properties of both metal and non-metal, and belongs to the p-block of group 14 of the periodic table. It is a grayish-white, lustrous, hard, brittle metal.
Germanium is the scarce element on the Earth’s crust.The average concentration of germanium on Earth is 14 parts per million (ppm), however, most of it is found in the core (37 ppm), with the crust only containing 1.5 ppm.
Interesting Science Videos
History of Germanium
- In 1871, Russian chemist Dmitri Mendeleyev hypothesized that several unidentified elements may exist due to the gaps in his newly created periodic table of elements.
- A new mineral, which was named argyrodite due to its high silver content, was discovered at a mine near Freiberg, Saxony, in the year 1885.
- When German Clemens Winkler, a chemist, examined this argyrodite, he discovered that it was a combination of sulfur, silver, and a completely undiscovered element.
- Germanium was discovered by the German chemist Clemens Winkler in the mineral argyrodite (Ag8GeS6) in 1886.
Occurrence of Germanium
- Germanium is a highly reactive element so it rarely occurs in nature. Only a small number of minerals, including argyrodite, briartite, germanite, renierite, and sphalerite, have significant concentrations of germanium.
- The amount of germanium in the Earth’s crust is comparable to that of beryllium, molybdenum, and cesium on a weight basis (approximately 1.5 parts per million).
- The majority of the germanium created is a by-product of the extraction procedures for sphalerite, coal, and other ores.The only sources of this element that are exploited economically are germanite and renierite.
- Germanium can also be extracted as a by-product from the combustion of some specific coals. China is the biggest producer of germanium , followed by Russia and the United States, among others.
Isotopes of Germanium
There are five stable isotopes of Germanium (Ge) that are present in nature:70Ge,72Ge 73Ge 74Ge and 76Ge
| Isotope | Natural abundance (atom %) |
|---|---|
| 70Ge | 20.84 (87) |
| 72Ge | 27.54 (34) |
| 73Ge | 7.73 (5) |
| 74Ge | 36.28 (73) |
| 76Ge | 7.61 (38) |
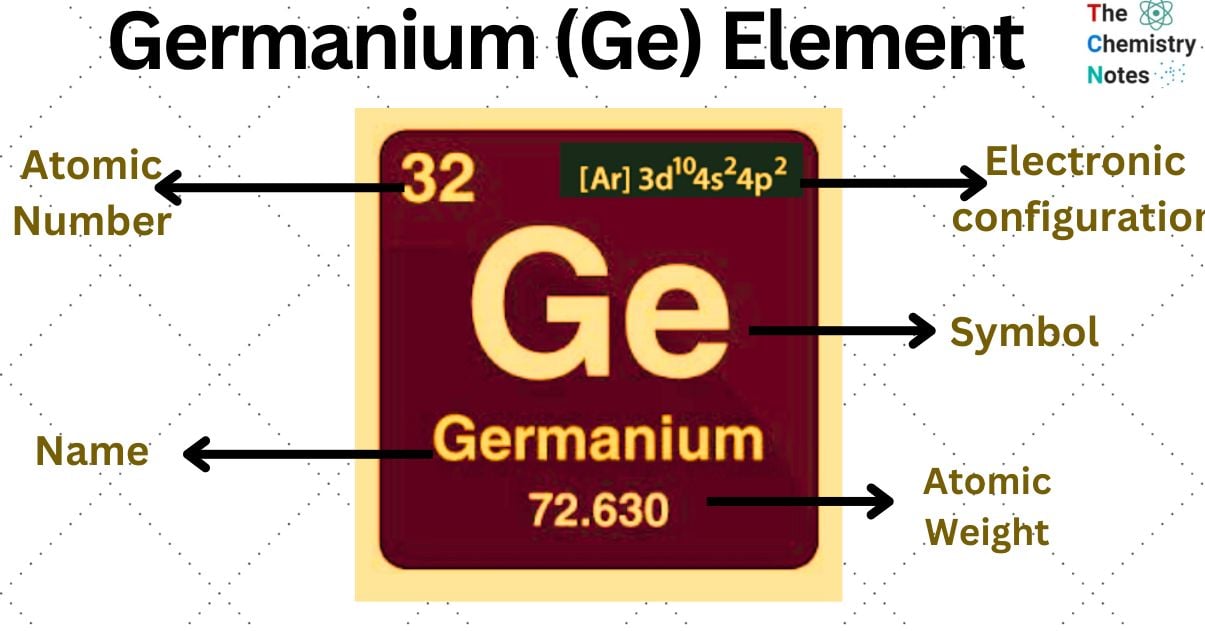
Elemental Properties of Germanium
| Electronic Configuration | [Ar] 3d104s24p2 |
| Atomic Number | 32 |
| Atomic Weight | 72.630 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 14, 4, p-block |
| Density | 5.3234 g.cm -3 at 20 °C |
| Ionic radius | 0.093 nm (+2) ; 0.054 nm (+4) |
| Van der Waals radius | 0.137 nm |
| Electron shells | 2, 8, 18, 4 |
| Electrons | 32 |
| Protons | 32 |
| Neutrons in most abundant isotope | 42 |
Physical Properties of Germanium
- Germanium has an atomic number of 32 and is a hard brittle silvery metalloid.It has a melting point of 938.25°C (1720.85°F) and a boiling point of (2833°C, 5131°F).
- The density of Germanium is 5.32 grams per cubic centimeter.
- Germanium exists as a solid with a diamond-shaped crystal structure.
- It has a semiconductor properties; electrical and semiconducting properties of germanium are equivalent to those of silicon. It can become superconductor in the presence of strong electromagnetic field.
- Germanium also has the strange feature of expanding as it freezes (similar to water).
Silicon, bismuth, antimony, and gallium are four more elements that expand when frozen. - It has a bitter taste but has no odor.
- Germanium has a low toxicity.
| Color/physical appearance | gray-white |
| Melting point/freezing point | 938.25°C, 1720.85°F, 1211.4 K |
| Boiling point | 2833°C, 5131°F, 3106 K |
| Density | 5.3234 g cm-3 at 20°C |
| Malleability | No |
| Ductility | No |
Chemical Properties of Germanium
Germanium is a relatively inactive element.It doesn’t react with water and oxygen at room temperature. At higher temperatures it can be reactive with oxygen and can dissolve in acids. When finely split, it becomes more active.
Chemical Reaction of Germanium
- Reaction of Nickel with Water
A very thin layer of germanium dioxide, GeO2, shields the outermost layer of a lump of germanium. It is slightly more reactive than silicon, which comes immediately above germanium in the periodic table. This oxide layer renders germanium water-insensitive.
- Reaction of Germanium with Air
At high temperatures, germanium combines with oxygen in the air to form germanium dioxide.
Ge(s) + O2(g) → GeO2(s)
- Reaction of Germanium with Halogens
At the temperature of 150-200 °C, Germanium reacts with chlorine to produce germanium (IV) chloride.
Ge + 2Cl2 → GeCl4
Germanium reacts with bromide to form Germanium bromide.
Ge + 2Br2 → GeBr4
Uses Of Germanium
Due to the high melting and boiling point germanium can be applicable in high temperatures. It is also a semiconductor which makes it important material for the electronics. There is a huge list of sectors where Germanium is applicable some of which include:
Used In Semiconductor Technologies: Apart from its usage in semiconductors, germanium has a wide range of other essential applications. For example, germanium is utilized in the production of solar energy cells because it enhances their efficiency. It is also utilized in the production of fiber optic cables to increase signal quality.
Used In Optical Components for Infrared Technology: Germanium has an exceptional capacity to absorb and emit infrared light, which makes it excellent for the production of infrared detectors and lenses. These components are used in night-vision goggles, thermal imaging cameras, and infrared astronomy.
Used In Transistors: Germanium is a great material for high-speed transistors due to its strong electron mobility. These transistors are utilized in a wide range of applications, including computer processors, communication systems, and radar systems.
Used In Optical Fibers: An optical fiber is an extremely thin thread made of pure glass that transmits data via light waves. The ability of a glass thread to convey light is influenced by impurities. To improve their ability to convey light messages, optical fibers are doped with germanium and other elements. Rather than utilizing electrical cables, optical fibers are now employed to transport telephone messages.
Used As A Catalyst: It acts as a catalyst in several polymerization processes. A catalyst is used to either speed up or slow down a chemical reaction. The catalyst undergoes no chemical alteration during the procedure. Germanium catalysts are commonly utilized in the polymer manufacturing process.
Used In Military Application: Germanium is also employed in the production of specialty glass for military applications. It is utilized to construct weapons-sighting systems that can be operated in the dark, for example. Satellite systems and fire alarm systems may also contain germanium-containing glass.
Health Effects Of Germanium
Germanium is not required by plants or animals. Because it occurs in such minute quantities in nature. Even though there are no scientific findings about the benefits of germanium to humans, a lot of germanium supplements claim to improve the O2 supply in the body, improve your immune system, and also destroy the free radicals that might affect your body and its proper functioning. However, some studies have connected side effects like muscle weakness, anemia, kidney damage, etc. to the use of these supplements.
Germanium dioxide does not irritate the skin, but when it comes into contact with wet conjunctiva, it produces germanic acid, which is an eye irritant. Long-term intra-abdominal injection of 10 mg/kg causes peripheral blood alterations.
Environmental Effects Of Germanium
Although germanium is not a toxic element, some evidence of its effect on aquatic life has been suggested by some research.
- The production of single crystals of germanium for the manufacture of semiconductors results in high air temperatures (up to 45 ºC), electromagnetic radiation with field strengths of more than 100 V/m, and magnetic radiation with field strengths of more than 25 A/m, and metal hydride pollution of the workplace air.
- Germanium hydride, which is used to produce high-purity germanium, may also be an occupational air pollutant. The regular cleaning of vertical furnaces results in the generation of dust, which comprises, in addition to germanium, silicon dioxide, antimony, and other chemicals.
- Dust is produced during the machining and grinding of germanium crystals. During dry machining, concentrations of up to 5 mg/m3 have been recorded.
- After surviving acute poisoning, animals develop catarrhal-desquamative bronchitis and interstitial pneumonia. Germanium chloride is also hazardous in general. The liver, kidneys, and other organs of the animals have undergone morphological modifications.
Do not repeat the experiments shown in this video! Watch the video of metalloid as germanium, which helps to immediately transfer information over thousands of kilometers, creating the modern Internet.
References
- Höll, R.; Kling, M.; Schroll, E. (2007). “Metallogenesis of germanium – A review”. Ore Geology Reviews. 30 (3–4): doi:10.1016/j.oregeorev.2005.07.034.
- https://www.rsc.org/periodic-table/element/32/germanium
- https://www.lenntech.com/periodic/elements/ge.htm
- https://pubchem.ncbi.nlm.nih.gov/element/Germanium
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- https://www.chemicool.com/elements/germanium.html
- Tao, S. H.; Bolger, P. M. (June 1997). “Hazard Assessment of Germanium Supplements”. Regulatory Toxicology and Pharmacology. doi:10.1006/rtph.1997.1098. PMID 9237323
- Gerber, G. B.; Léonard, A. (1997). “Mutagenicity, carcinogenicity and teratogenicity of germanium compounds”. Regulatory Toxicology and Pharmacology. doi:10.1016/S1383-5742(97)00034-3. PMID 9439710

