Gel filtration chromatography, also widely recognized as size exclusion chromatography, is a versatile technique for separating proteins and other biological molecules. Gel filtration chromatography separates proteins solely based on molecular size variations. The alternate names for gel filtration chromatography are size exclusion, molecular sieve, and gel permeation chromatography.
What is Gel-filtration Chromatography?
In analytical chemistry, gel chromatography, also known as gel filtration chromatography, is a technique for separating chemical substances by taking advantage of the variations in the rates at which they pass through a bed of a porous, semisolid substance.
The technique is particularly helpful for separating proteins, peptides, amino acids, enzymes, and other molecules with low molecular weight from one another. By using gel chromatography, the components of a mixture can be separated based on the variations in their molecular sizes.
In other words, gel filtration chromatography is a type of chromatography that separates components based on their molecular sizes using porous gel beads of a particular porosity. The primary factors used in this method to retain or exclude particles are size differences, hydrophobicity, and molecular charges.
Principle of Gel-filtration Chromatography
The fundamental idea behind gel filtration chromatography is that the components of a mixture can be separated based on how different molecules are sized.
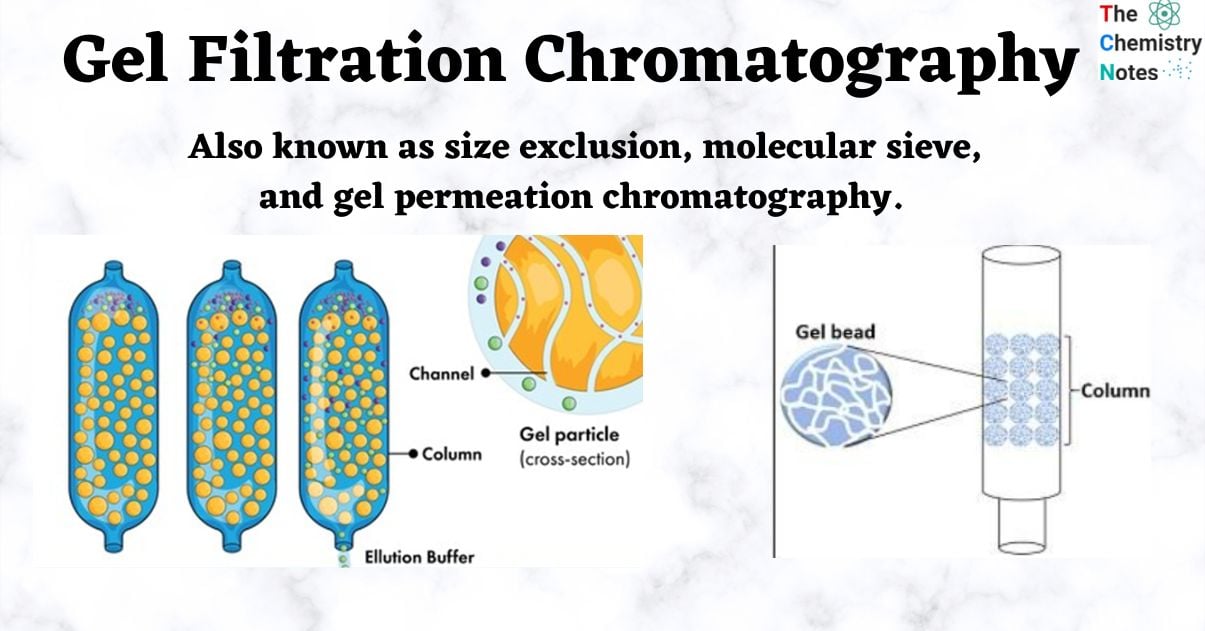
- Gel filtration chromatography is a type of chromatography that separates components based on their molecular sizes using porous gel beads of a particular porosity.
- Depending on the elution limit, some molecules elute earlier or later through the column as the components of a liquid mixture pass through it.
- Small molecules have a tendency to diffuse into the interior of porous particles, which restricts their flow, whereas large molecules are unable to enter the pores and have a tendency to flow freely.
- The highest molecular weight components, therefore, leave the bed first, followed by successively smaller molecules.
The elution limit is a variable that affects whether molecules pass through the packing material and are retained or excluded. It primarily aids in the isolation of biomolecules such as proteins, peptides, and oligonucleotides.
Phases in Gel Filtration Chromatography
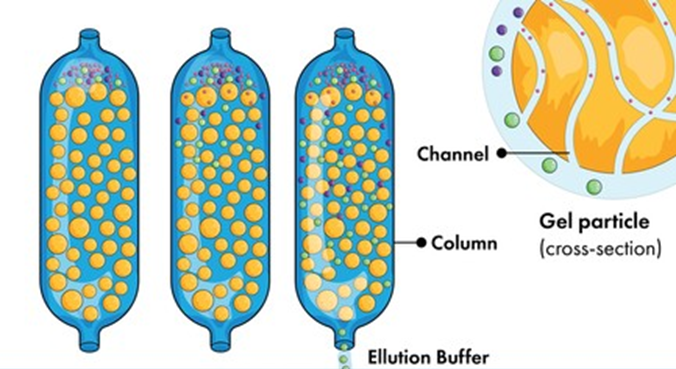
Mobile phase: The solvent that is circulating through the column is the mobile phase. To begin, the test sample must be diluted in appropriate organic solvents. Next, filter the test sample and run it through the column. In the column, a combination of many components separates.
Stationary Phase: Microporous gel beads are arranged in a column, which serves as the stationary phase. Gel filtration chromatography frequently uses cross-linked polyacrylamide, agarose gel, and hydroxypropylated Sephadex as support materials.;
Procedure/Steps in Gel-filtration Chromatography
- The first step in the gel filtration chromatography process is to prepare a column of spherical gel beads.
- A buffer is used to balance the packed bed.
- The test solution (mobile phase) is then eluted through the column.
- The particles in the test sample will then diffuse into or into the porous gel matrix after that (stationary phase).
- Small molecules will enter the gel pores, resulting in a longer path or staying time on the column.
- Large molecules with large molecular sizes cannot easily pass through the column or into the pores of gel beads.
- As a result, after the components have been isolated and identified, the particles are separated at various points.
Any molecule or complex that is above a given gel filtration chromatography column’s fractionation range will pass through the column faster than any molecule that can enter the stationary phase. Any component in the sample that is above the fractionation range will, therefore, elute first (in the void volume) before any component that is within the fractionation range. The exclusion limit is the smallest size that will stay in the mobile phase and avoid going into the stationary phase.
Applications of Gel Filtration Chromatography
One of the primary advantages of gel-filtration chromatography is that separation can be performed under conditions that are specifically designed to maintain the stability and activity of the molecule of interest without compromising resolution. Because of its simplicity, dependability, versatility, and ease of scale-up, gel-filtration chromatography holds a key position in the field of biomolecule separation.
Protein and peptide separation
Thousands of proteins and peptides from various sources have been successfully purified using gel-filtration chromatography due to their distinctive mode of separation. These include industrial enzymes and proteins, as well as therapeutic proteins and peptides.
Separations of the Groups
One can achieve a group separation that would typically require up to 24 hours of dialysis by choosing a matrix pore size that entirely excludes all of the bigger molecules in a sample from the internal bead volume while making it easy for extremely tiny molecules to enter this volume. Group separation can be used to remove phenol from nucleic acid preparations, effect buffer exchanges within samples, desalt labile materials prior to concentration and lyophilization, and remove inhibitors from enzymes
Nucleic acid and nucleotide separation
For many years, different nucleic acid species, including DNA, RNA, and tRNA, as well as their component bases, adenine, guanine, thymine, cytosine, and uracil, have been separated using gel- filtration chromatography.
Application with Specific example
- By using urea-gradient size-exclusion chromatography on a Superdex 75 column, recombinant human granulocyte colony-stimulating factor (rhG-CSF) was efficiently refolded from inclusion bodies while significantly suppressing the formation of aggregates. Alpha-lactalbumin and beta-lactoglobulin, two whey proteins, can be separated from aqueous two-phase systems using gel filtration.
- Examining the metal ion-mediated assembly of protein cages is made easier by the gel-filtration method.
- By using gel filtration on Sephacryl S-1000 Superfine, it is possible to completely separate phage DNA from chromosomal DNA and RNA, such as circular double-stranded phage M13 DNA and linear phage lambda DNA.
- The use of Sephacryl S-1000 gel-filtration chromatography resulted in more effective purification of turkey coronavirus from infected turkey embryos than the use of a sucrose gradient.
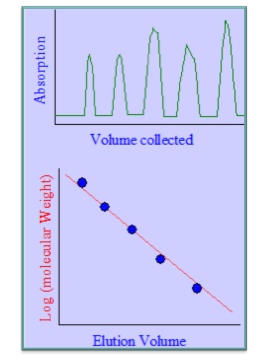
- Gel-filtration chromatography is an ideal alternative to SDS-PAGE for determining the relative molecular weights of proteins since the elution volume of a globular protein is directly linked to the logarithm of its molecular weight.
Estimation of Molecular Weight
Size affects how an analyte is distributed across the stationary and mobile phases. Its equilibrium distribution coefficient serves as a descriptional parameter (Kd). Kd is the proportion between the component concentrations in the stationary and mobile phases. Kd = 0 for component A since it is unable to enter the stationary matrix’s pores. Kd for component B may be expressed as follows:
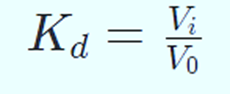
Vi is the overall volume of the beads’ pores that is accessible to solvents.
The Kd value increases with particle size because smaller particles may penetrate the beads’ pores more readily. Kd is thus a function of particle size. To estimate particle size, Kd must be determined.
Advantages of Gel Filtration Chromatography
- The renaturation of denatured proteins is made possible using a gel filtration system.
- It supports investigations involving protein fractionation.
- With the use of gel filtration chromatography, the molecular weight of the separated particles could be determined.
- This technique looks at the quaternary structure of isolated proteins as well.
- Biomolecules that are sensitive to changes in pH, temperature, metal ion concentration, etc. can be separated via gel filtration.
- In Ion exchange chromatography, the particles do not adhere to the chromatography medium.
- A quick explanation of the outcome is required.
- It creates a distinct divide.
- To draw conclusions about the outcomes, only a tiny test sample is required.
- The flow rate might be altered.
Limitations/Disadvantages of Gel Filtration Chromatography
There are several drawbacks to this strategy, though. Proteolysis, for instance, becomes a bigger issue when separating proteins using gel-filtration chromatography since the target protein typically serves as an abundant substrate for proteases that are also present in the mixture, which lowers the recovery of activity.
- Large amounts of eluent are frequently needed for gel-filtration columns to function because of their size, which results in high operating expenses.
- As opposed to other chromatographic methods, gel filtration has an intrinsic poor resolution since none of the molecules are retained by the column, and non-ideal flow occurs around the beads.
- Additionally, this method’s poor sample-handling capacity is a result of the requirement to maximize resolution.
- The restricted number of peaks can be resolved during the run’s short time frame.
- Before utilizing the instrument, filters must be run to stop dust and other pollutants from damaging the columns and interfering with the detectors.
- Most chains’ molecular weights will be too similar for the separation to display anything other than wide peaks.
References
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- Mallik B, Chakravarti B, and Chakravarti DN (2012). Overview of Chromatography. In: Gallagher SR and Wiley EA, editors. Current Protocols Essential Laboratory Techniques, 2nd Edition. Wiley-Blackwell.
- https://bitesizebio.com/29685/determine-molecular-weight-gel-filtration-chromatogram/
- https://biologyreader.com/gel-filtration-chromatography.html#Phases
- Tepfer., & Taylor., E. I. (1981). The permeability of plant cell walls as measured by gel filtration chromatography. Science, 213(4509), 761-3.
- L. Andersson., W. T. Barrowcliffe., E. Holmer., A. E. Johnson., & Sims., G. E. (1976). Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin iii and by gel filtration. Thromb Res, 9(6), 575-83.
- https://microbeonline.com/gel-filtration-chromatography/
