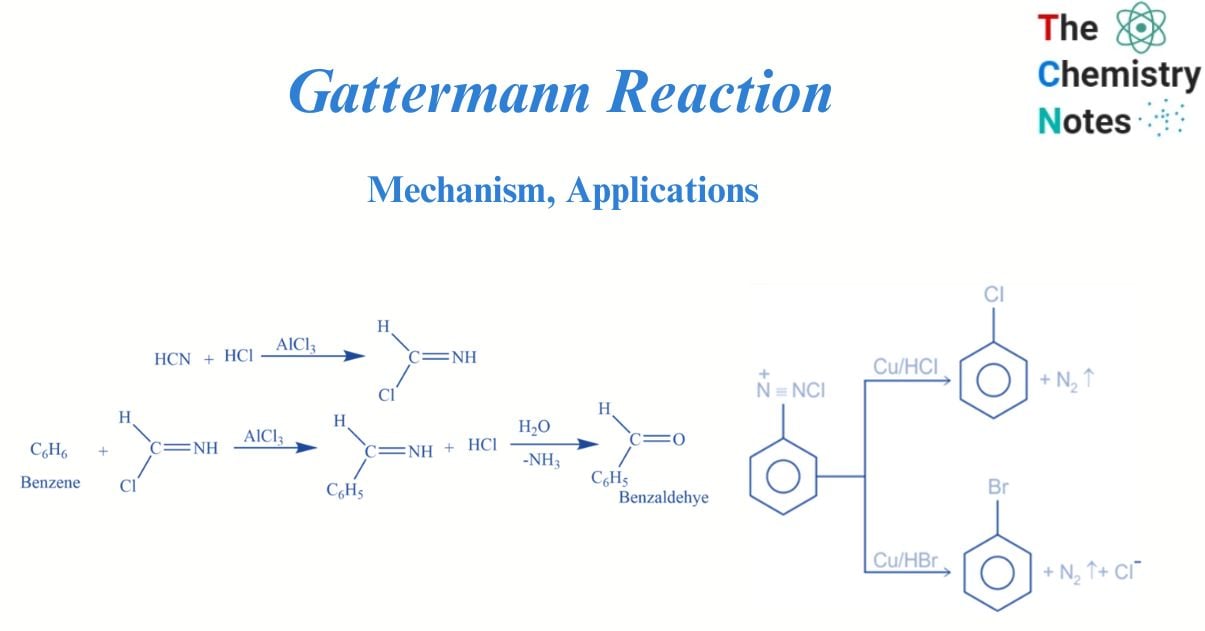
The Gattermann reaction is an organic reaction that involves the formylation of aromatic compounds by a reaction of hydrogen cyanide and hydrochloric acid (HCl) in the presence of a Lewis acid catalyst such as AlCl3. Formylation is the process of attaching the formyl group (-CH=O) to a molecule. It is also known as the Gattermann formylation reaction or the Gattermann salicylaldehyde synthesis reaction. This reaction is named after a German chemist named Ludwig Gattermann, and it has many similarities to the Friedel-Crafts reaction.
It’s utilized to make aromatic ring compounds like aromatic halides and aromatic aldehydes.
Interesting Science Videos
What is Gattermann’s reaction?
Benzene reacts with a mixture of hydrogen cyanide and hydrogen chloride in the presence of aluminium chloride to produce benzaldehyde.
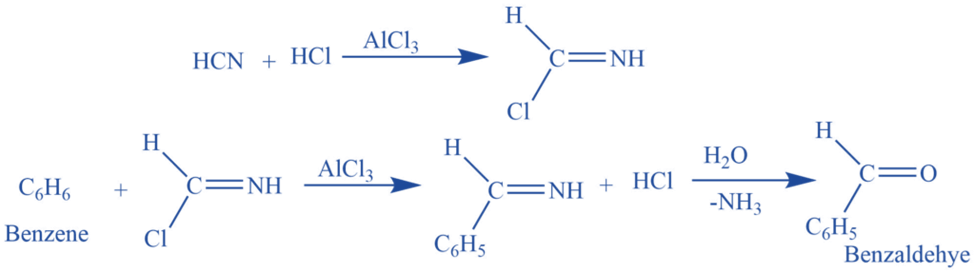
Mechanism of Gattermann reaction
Step 1: Formimino Chloride Formation: HCN reacts with HCl to generate formimino chloride.
Step 2: Electrophile Formation: Formimino chloride combines with a Lewis acid catalyst like Alcl3 to form Formimino cation.
Step 3: Electrophile Attack on the Benzene Ring: The formimino Cation combines with the benzene ring to produce Benzaldimine.
Step 4: Benzaldimine Hydrolysis: Benzaldimine is hydrolyzed, resulting in the formation of benzaldehyde.
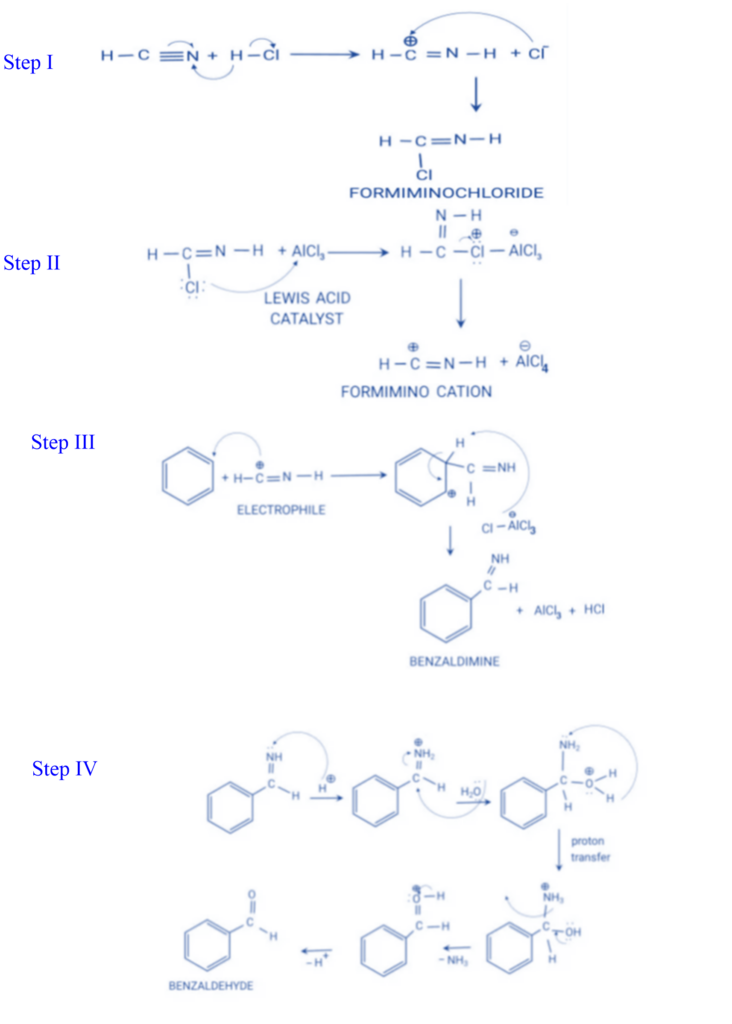
Preparation of chlorobenzene and bromobenzene by using Gattermann reaction
A variation of the Sandmeyer reaction is the Gattermann reaction. When a diazonium salt is heated with cuprous chloride in hydrochloric acid or cuprous bromide in hydrobromic acid, the corresponding halide is produced in the Sandmeyer reaction. The Sandmeyer reaction was modified by Ludwig Gattermann. In this reaction, the diazonium salt is heated with copper powder and the appropriate halogen acid to form corresponding halogen derivatives.
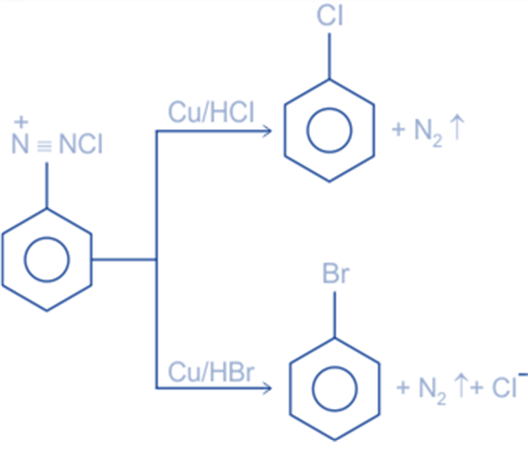
The Gattermann reaction cannot be used to create iodobenzene or fluorobenzene. This is because iodine is an oxidizing agent and combines with itself to produce I2, while fluorine is a highly exothermic gas, making it unable to react with fluorine at room temperature.
Application of Gattermann reaction
- It is used to produce aromatic halides such as chlorobenzene and bromobenzene.
- It is utilized in the production of aromatic aldehydes such as Benzaldehyde.
- Gattermann reaction products such as benzaldehydes, haloarenes, chlorobenzene, and others are used in a variety of industries including pharmaceuticals, agriculture, and medicine.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
