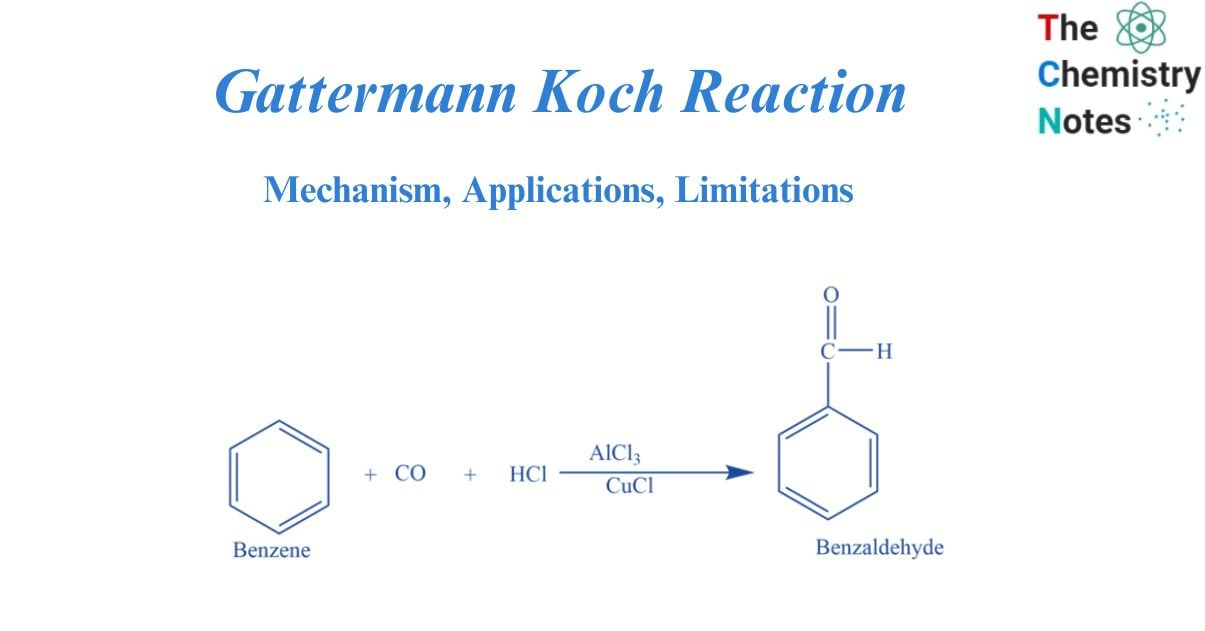
Gattermann Koch reaction involves the reaction of benzene with CO and HCl in the presence of Lewis catalysts e.g., AlCl3 to form benzaldehyde.
In 1897, L. Gattermann and J.A. Koch developed this reaction process. They successfully added an aldehyde group to toluene by using formyl chloride (HCOCl) and Lewis catalysts (e.g., AlCl3, FeCl3, etc.)
The Gattermann-Koch reaction is a variation of the Gattermann chemical reaction that uses carbon monoxide in place of hydrogen cyanide. It is an illustration of an electrophilic substitution process. Here, anhydrous aluminum chloride or cuprous chloride is present as catalysts for the reaction between benzene and carbon monoxide in an acidic media.
The Gattermann Koch reaction does not work for phenol and phenol ether substrates, in contrast to the Gattermann reaction. Formyl cation, or protonated carbon monoxide, [HCO] +, is currently believed to react directly with the arene without the initial Gattermann-Koch reaction production of formyl chloride, despite the exceedingly unstable formyl chloride initially being hypothesized as an intermediary.
Furthermore, when zinc chloride is employed as the catalyst, or when carbon monoxide is not used at high pressure, traces of copper(I) chloride or nickel(II) chloride cocatalyst are frequently required. The transition metal co-catalyst can operate as a “carrier” by first interacting with CO to generate a carbonyl complex, which is subsequently converted into the active electrophile.
Interesting Science Videos
What is Gattermann Koch’s Reaction?
In the presence of anhydrous aluminium chloride and traces of copper (I) chloride, benzene reacts with carbon monoxide and hydrochloric acid to produce benzaldehyde. This reaction is known as Gattermann -Koch reaction. Here copper (I) chloride acts as a co-catalyst.
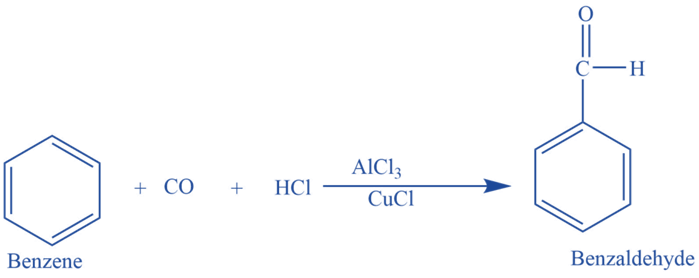
Reaction mechanism of Gattermann Koch Reaction
Step I: Due to its nucleophilic properties, carbon monoxide attacks HCl molecules, which results in the reaction intermediate with two resonance structures. The oxygen atom’s enormous size prevents Cl- from attacking it so electrophilic Cl- ion attacks the carbon atom of the intermediate. This reaction produces Formyl Chloride (HOCl).
Step II: AlCl3 frequently reacts with a Cl- ion to generate the [AlCl4]- complex. As a result, the Cl atom in formyl chloride attacks alkyl chloride (AlCl3). Once again forming the formyl cation, which is now free to interact with aromatic molecules.
Step III: The presence of π-electrons makes the benzene electron-rich. The electrophilic carbon in the formyl cation is attacked by the benzene ring, which functions as a nucleophile. A “σ-complex” is created as a result of this. Through a reaction with the σ-complex, the [AlCl4]- generated during the preceding step is changed back into AlCl3. The final product is stable benzaldehyde.
Applications of Gattermann Koch Reaction
- Alkylbenzenes are converted into aldehydes via this process.
- Gattermann Koch reaction is an industrial method used in the production of benzaldehyde.
Limitation Gattermann Koch Reaction
Due to a lack of suitable substrates, the Gattermann-Koch reaction can add a formyl group to alkylbenzenes; therefore, Gattermann came up with a modification that enables the formylation of phenols, phenolic ethers, and heteroaromatic compounds like pyrroles and indoles.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s advanced organic chemistry: reactions mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992.
