Gas-Liquid Chromatography (GLC) is a separation methodology that employs a gaseous mobile phase, typically an inert gas like helium or a nonreactive gas like nitrogen, and a liquid stationary phase. The fundamental principle underlying separation is based on the difference in partition coefficients exhibited by volatilized compounds between the gaseous and liquid phases. This phenomenon is observed when the desired compound is carried through the column by a carrier gas. The testing of the purity of a substance is an example of a typical application of Gas-Liquid Chromatography, as is the preparation of novel compounds from mixtures (known as preparative chromatography) and the identification and separation of the various components of a mixture.
It is also known as gas-liquid partition chromatography (GLPC) and vapor-phase chromatography (VPC). Both of these names refer to the same technique, gas-liquid chromatography.
Introduction to Gas-Liquid Chromatography
In the field of analytical chemistry, one of the most helpful methods is known as Gas-Liquid Chromatography or GLC for short. In 1946, Claesson noted what is considered to be one of the earliest significant accounts of gas-liquid chromatography. In the type of chromatography known as Gas-Liquid Chromatography, the stationary phase is a film that has been coated on a solid support, and the mobile phase is an inert gas such as nitrogen (N2) that is called a carrier gas and flows over the surface of a liquid film in a regulated manner. This type of chromatography is a form of partition chromatography. The sample that is going to be analyzed is vaporized while the settings involve programming a high temperature. The components of the vaporized sample are separated into their individual components as a result of a partitioning process that takes place between a mobile gaseous phase and a liquid stationary phase that is contained within a column.
Principle of Gas-Liquid Chromatography
The partitioning of chemicals between stationary liquid and mobile gas phase is the fundamental concept behind Gas-Liquid Chromatography. It is widely used for a variety of qualitative and quantitative studies due to its great sensitivity, repeatability, and speed of resolution. The gaseous chemicals that are being investigated have an interaction with the walls of the column, which are covered with various types of stationary phases. This results in each compound eluting at a distinct time, which is referred to as its retention time. The retention times are then compared. Because of this, Gas-Liquid Chromatography is a very valuable analytical tool. When a compound is removed from the column, it goes via a detector on its way out. This detector is connected to a chart recorder through an amplifier. The chart recorder keeps track of the highest points.
The separation of various components of a sample mixture occurs based on their partition coefficient between the gas and liquid stationary phase, as the vapors of the mixture move between these phases.
It is a generally accepted principle that the rate of component emergence is proportional to the value of the partition coefficient, and vice versa. The compounds that have a low boiling point (B.P), also known as having a higher volatility and vapor pressure, will have a larger concentration in the mobile phase, and as a result, they will elute or emerge first, and so on. For instance, compounds with a lower carbon number have a lower boiling point and higher volatility and vapor pressure, hence they will elute first than compounds with a higher carbon number. For instance, shorter chain lengths of fatty acids emerge first, followed by longer chain lengths. Therefore, compounds with less polarity elute more quickly than those with more polarity. When compared to less polar compounds, which move at a faster rate due to their lower rate of retention in the column, the movement of more polar substances causes the column to move more slowly.
In chromatographic analysis, the words “retention time” and “retention volume” are among the most frequently encountered.
- Retention time: It is the amount of time that must pass in order for the maximum of a solute peak to arrive at the detector in a gas chromatographic column. This peak represents the specific component being analyzed. The retention time, denoted by tR, is indicative of that component, and the area under the peak is directly proportional to the component’s concentration. These characteristics, used together, produce both qualitative and quantitative data, respectively.
- Retention volume: The term “retention volume” (VR) refers to the amount of gas that must be present in order to transport a component’s maximal concentration through the column. [VR = tR Fc Where Fc is the volume flow rate of the gas at outlet[.
Instrumentation of gas-liquid chromatography
The main components of gas-liquid chromatography are:
Carrier gas supply
It is imperative that the mobile phase in its gaseous state exhibits inertness. The most frequently utilized mobile phase in gas chromatography is helium, although argon, nitrogen, and hydrogen are also employed. The majority of these gases, which are highly pure in nature, are obtainable in cylinders, including mixtures like nitrogen and hydrogen.
In gas chromatography, it is imperative to ensure that the gases employed are adequately dehydrated, as the presence of moisture within the gases can result in undesirable background noise. Currently, suppliers of gas chromatography equipment are offering desiccant cartridges and additional filters to address issues related to impurities and other contaminants. Alternatively, the optimal choice for a desiccant would be a molecular sieve (specifically Linde 5A) that has undergone activation at a temperature range of 200 to 300 degrees Celsius. The regulation of gas flow rate is facilitated through the utilization of pressure gauges and flow meters.
Sample injection system
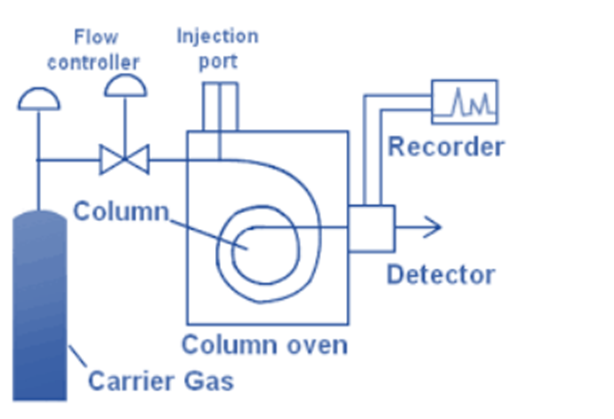
The technique for sample injection is dependent upon the nature of the sample, whether it is gaseous, liquid, or solid. The Gas-Liquid Chromatography protocol stipulates that an appropriate quantity of sample must be introduced as a condensed mass of vapors. Band broadening and suboptimal resolution have been observed as a result of slow injection or the use of excessively large samples. The appropriate sample size is contingent upon the detector’s sensitivity. In the case of utilizing an ionization detector, it is recommended that the liquid sample size does not exceed 0.5 µL.
- Liquid samples: The introduction of liquids is achieved via micro syringes through a silicon septum and directed towards a heated sample port situated at the head of the column. Typically, the temperature of the sample port is maintained at a level that is at least 50 degrees Celsius higher than the boiling point of the least volatile constituents present in the sample. In the case of conventional packed analytical columns, the sample sizes typically vary from a few tenths of a microliter to 20 microliters. Capillary columns require samples that are reduced in size by a magnitude of 100 or greater. In many cases, it is necessary to utilize a splitter to dispense a predetermined and minimal fraction (ranging from 1:100 to 1:500) of the injected sample, while the remaining portion is discarded.
- Solid samples: Typically, solid samples are quantified by transferring them into thin glass ampoules, which are subsequently introduced into the gaseous flow and subsequently fragmented.
Columns
The effectiveness of a gas chromatograph is significantly influenced by the type of columns utilized in Gas-Liquid Chromatography(GLC), which can be constructed from either glass or metal materials. The columns in question can be broadly classified into two categories, namely packed columns and open-tubular (capillary) columns.
- Packed column: The earlier mentioned columns possess the ability to accommodate larger samples and are typically deemed more convenient for utilization. Typically, they measure between 2 to 3 meters in length and possess internal diameters ranging from 2 to 4 millimeters. Typically, the tubes are configured in a coiled shape with a diameter of approximately 15cm to facilitate easy thermal treatment within an oven. The function of the column’s packing or support is to immobilize the liquid stationary phase, thereby maximizing the surface area available for interaction with the mobile phase. The optimal particle size for packing material in gas chromatography falls within the range of 60-80 mesh (250-170 μm) or 80-100 mesh (170-149 μm).
- Capillary columns: Capillary columns are typically composed of either glass or fused silica. The aforementioned columns possess internal diameters ranging from 0.25 to 0.50 mm and lengths spanning from 25 to 100 m. Silica capillaries possess thinner walls in comparison to their glass or metal equivalents and exhibit outer diameters of approximately 0.3 mm.
- Column oven: The oven utilized in gas-liquid chromatography (GLC) typically features a high-precision thermostat, which serves to regulate the temperature of the column housed within the oven. This is done in order to achieve consistent and replicable retention times. The temperature range exhibits variability within the interval of 0 to 400 degrees Celsius.
Detectors
The detectors are extremely sensitive and have a rapid response time, allowing them to detect even the most minute quantities of solutes as they leave the columns. The linear response, stability, and uniform response are all possessed by detectors for a large variety of chemical species. There is a wide variety of detectors that can be chosen from. (a) The conductivity of heat (b) The density of gases (c) Ionization caused by a flame (d) ionization caused by beta rays (cross-section, argon, helium, electron capture, and electron mobility) (e) ionization caused by light. (f) Glow discharge (g) Temperature (h) of the flame Dielectric constant. The two detectors described above are the ones that find the most widespread application.
Thermal conductivity detector
Another designation for this instrument is the katharometer. This is composed of a network of electrical bridges made up of four filaments arranged in a specific pattern. The carrier gas that is flowing around and through the cavities of these filaments. The rate of heat loss that occurs due to conduction through the carrier gas is what ultimately decides the temperature of the filament. Changes in the components that elute out of the column cause changes in the composition of the gas, which in turn causes variations in the thermal conductivity. Because of this, there is a subsequent change in the temperature of the filaments, which results in electrical output from the bridge circuit. Its strengths include (i) its ease of use and (ii) its versatility. iii) it does not cause any damage, making it ideal for use in the pre-collection of fractions. Having a low resistance and sensitivity (10 -9 g/mL carrier) are both problems.
Flame Ionization detector
The most often used detector because of its superior sensitivity, range, and dependability. Only organic substances will elicit a reaction from it. Most organic compounds form ionic intermediates that conduct electricity when they pyrolyze in a hot flame, which is how this device operates. A small amount of hydrogen is being burned in an overabundance of air, and this is all encased in an electrostatic field. Hydrogen entering the burner is combined with the effluent from the column. Carbon dioxide is generated by the combustion of organic materials eluted from the column. Some ionizing particles and electrons are generated as intermediate products of oxidation during this process. The quantity of these ionizing particles is directly related to the quantity of carbon in the initial molecules. The polarizing electrodes capture and neutralize these ionizing particles, resulting in an electric current that is measured by an electrometer as a peak. The ionization detector has a broad linear response, low noise, and excellent sensitivity (1013 g/mL). It’s sturdy and simple to operate, too. It can only react to organic substances. Light hydrocarbon gases have a detection limit of 5 ppb, while higher organic liquids and gases require 10 picograms for detection. The detectable amount drops to around 1 nanogram (107 – 108 g) at column temperatures of 200 °C or higher. One of the major disadvantages is that it destroys the sample.
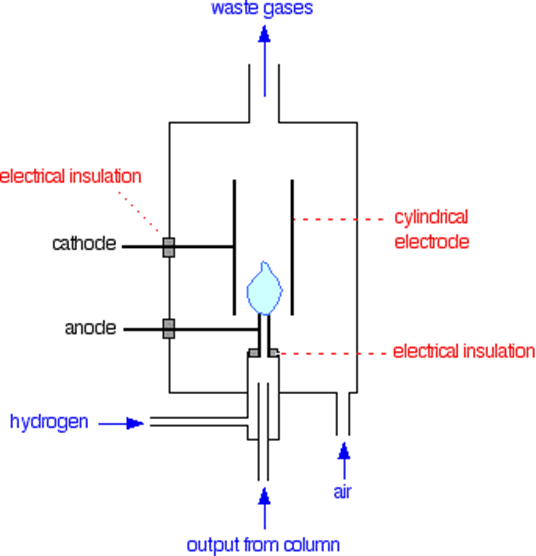
Figure: Flame ionization detector (Source: https://chem.libretexts.org)
Modified Flame ionization detector (FID)
Thermo ionic alkali flame detector (TID) is another name for it. To increase the ionization of compounds containing P. Cl and N., an alkali metal salt is added to the flame. By means of (i) a fixed wire (ii) a heated capillary (iii) a pallet fastened on a jet, the salts, primarily PO4 and halides of Na, K, Rb, or CS, are fed to the flame. When salts are used up, they must be refilled.
Electron capture detector
It works on the principle that different substances capture electrons in different ways, resulting in a diminished ion current. Electronegative chemicals have a high electron affinity because of the presence of electronegative elements or groups. The gaseous form favors the capture of electrons, leading to the formation of negative ions. A very sensitive selective detector for such compounds can be built by exposing them to a source of low-energy electrons. Tritium (H3)and Ni63 are two examples of radioactive sources used in ECD. The energy can also be lowered by using a quench gas, such as methane or argon (combined with a carrier gas). The advantages include a high level of sensitivity and specificity for molecules with strong electron affinities, such as oxygen, halogens, and compounds containing oxygen and halogens.
Nitrogen Phosphorus Detector (NPD)
This detector has a very high level of sensitivity and is a very specialized instrument. It demonstrates a high degree of sensitivity to organic molecules that either include nitrogen or phosphorus. The sensor in this detector consists of a bead made of rubidium or cesium that is housed inside a relatively small heater coil. The anode is located on one side of the detector. When heated, the alkali rubidium sulfate emits electrons through a process known as thermionic emission. These electrons are collected at the anode, which results in the production of ions. When a solute that contains nitrogen or phosphorus is eluted, the nitrogen and phosphorus materials that have been partially burned are adsorbed on the surface of the bead. Because of this deposited material, the work function of the surface is decreased; as a direct result of this, the emission of electrons is raised, which in turn enhances the anode current. The NPD has a detection limit of approximately 10-12 g/ml for phosphorus and 10-11 g/ml for nitrogen.
Interpreting output from the detector
The resulting data will be documented as a sequence of peaks, with each peak denoting a distinct compound within the mixture that has traversed the detector. Assuming that the conditions on the column were carefully controlled, the retention times could be utilized to facilitate the identification of the compounds that are present. This is, of course, contingent upon the prior measurement of pure samples of the various compounds under identical conditions by oneself or another individual.
The peaks can also serve as a means of quantifying the relative amounts of the compounds that are present. This assertion holds true solely in cases where the analysis pertains to mixtures comprising analogous compounds, such as hydrocarbons that are similar in nature. The proportional relationship between the areas under the peaks and the quantity of each compound that has traversed the detector is a notable feature. The computer that is connected to the display can perform automatic calculations of these areas. The depicted diagram presents a highly simplified illustration of the green-colored regions that would be assessed.
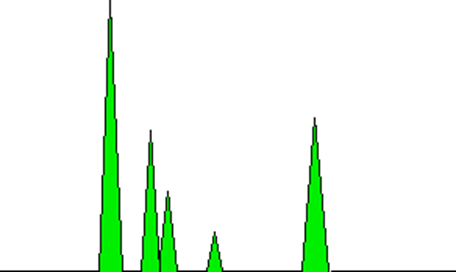
It should be noted that the significance lies not in the maximum elevation of the peak, but rather in the aggregate area encompassed by the peak. In this specific instance, the peak located on the left side exhibits the highest elevation and possesses the largest surface area. This assertion may not be universally applicable. Although a significant quantity of a particular compound could be present, it may be eluted from the column in relatively minute quantities over an extended duration. Utilizing the measurement of the area, as opposed to the height of the peak, facilitates this process.
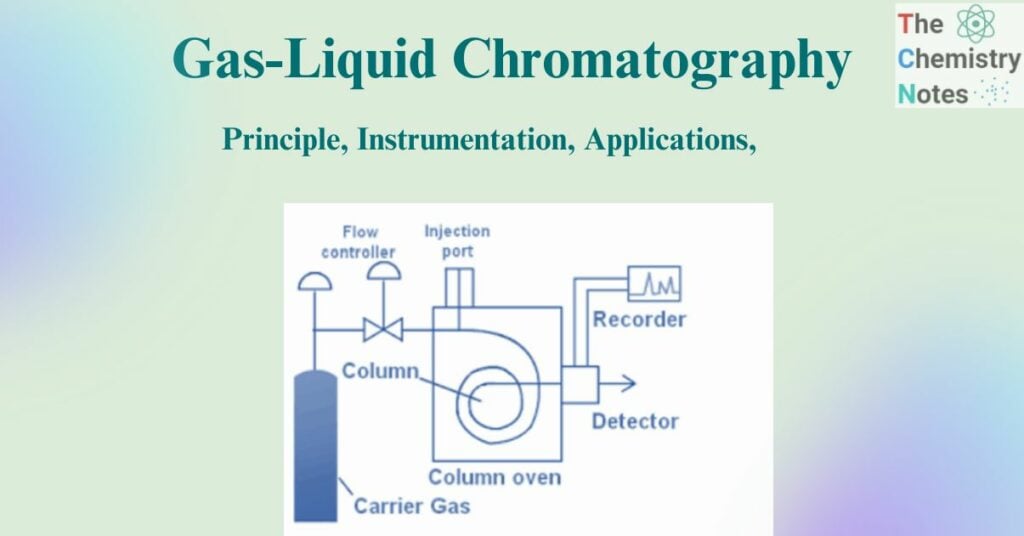
The standard illustration for the instrumentation of gas-liquid chromatography can be depicted as:
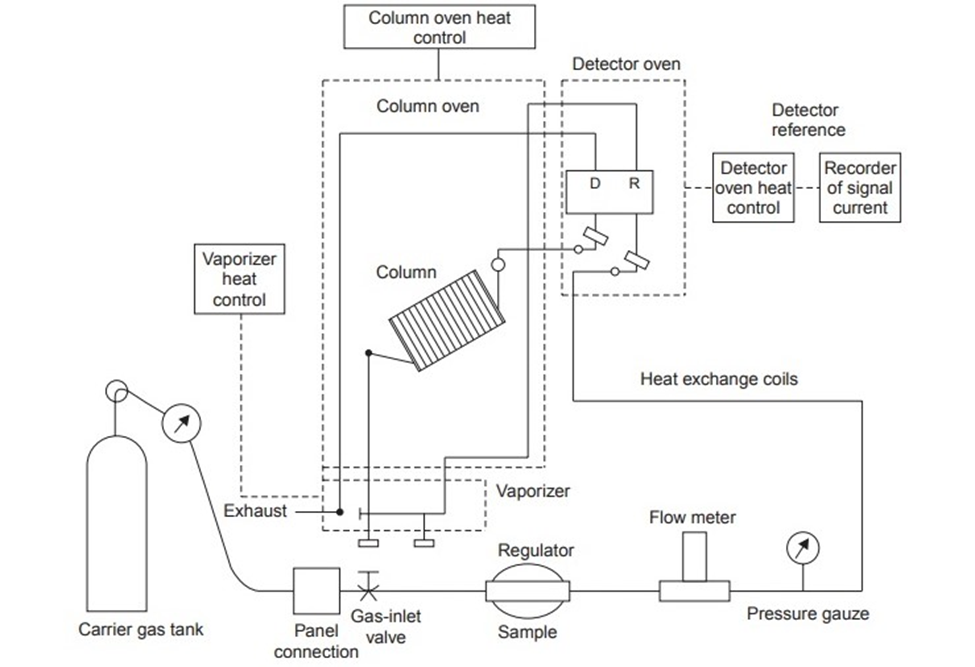
Figure: Standard Instrumentation of gas-liquid chromatography (Source: https://www.brainkart.com/article/Gas-Liquid-Chromatography-%28GLC%29–Instrumentation_30940/)
Stationary Phase for Gas-Liquid Chromatography
In the process of developing Gas-Liquid Chromatography, hundreds of different liquids have been suggested for use as stationary phases. The polarity of a liquid phase and the temperature range in which it can function well are two of the most significant elements to consider when choosing one. The latter can be ascertained through the use of tables or the provider. In a practical sense, the retention of a polar solute is larger than that of a non-polar solute with the same boiling point when the polarity of the liquid stationary phase is more than when it is lower. This is the case regardless of the boiling point of the solute. The rule states that similar things cancel each other out. In this case, a non-polar liquid phase is preferable since the components of the sample share a similar chemical structure but have distinct volatilities. If, on the other hand, the components have distinct functional groups but have comparable boiling temperatures, then the polar phase is typically the more appropriate choice. The majority of these stationary phases fall into one of two categories: polar or non-polar.
Polar stationary phase
They include functional groups such as – CN, – CO, and –OH in their composition. Polyethylene glycol and polyester, both of which are able to hold polar solutes, are included in these phases. These phases distinguish between different types of solute molecules based on their functional groups. The retention of carbon–carbon double bonds can be selectively shown by stationary phases that are selective. These phases are utilized for the purpose of separating carbonyls, lactones, and fatty acid esters.
Non-polar stationary phase
In general, they are of the hydrocarbon type (dialkyl siloxane), and some examples are methyl silicons, Apiezen greases, and squalene. They have a propensity to partition solutes according to B.P. Triglyceride is the term that is most commonly used.
Derivatives for Gas-Liquid Chromatography
The most frequently employed technique for derivatization involves its implementation prior to gas chromatography, thereby increasing the sample’s volatility.
- Methyl ester: Esterification reactions of fatty acids and fats employ both acidic and basic catalysts.
- Silyl ethers: Silyl ethers are the preferred option for the protection of partial glycerides, sterols, carbohydrates, and other related compounds.
- 2, 4-DNPH: Carbonyl compounds that exhibit volatility play a significant role as flavor constituents in various food products, contributing to both desirable and undesirable sensory attributes. The aforementioned entities are transformed into their respective 2, 4 dinitrophenyl hydrazones.
Coupling gas chromatogram to a mass spectrometer
The utilization of a flame ionization detector is not feasible as it has the propensity to destroy any substance that traverses its path. Presuming that a non-invasive sensor is being employed. Upon the manifestation of a peak on the detector, a portion of the substance traversing the detector can be redirected toward a mass spectrometer. The resulting fragmentation pattern can be cross-referenced with a computerized database of established patterns. This implies that the identification of a vast array of compounds can be accomplished without the requirement of prior knowledge regarding their retention times.
Applications of Gas-Liquid Chromatography
The separation and analysis of multicomponent mixtures, including essential oils, hydrocarbons, and solvents, are only some of the applications that can make extensive use of gas-liquid chromatography’s capabilities. It was a fundamental analytical method that was utilized in forensic laboratories all over the world. Some of the major applications are:
- In drug detection, fire investigation, environmental analysis, explosives investigation, and the identification of unknown substances, gas-liquid chromatography is frequently combined with mass spectrometry to develop the GC/MS technique.
- Gas chromatography-mass spectrometry (GC/MS) is utilized in airport security protocols for the identification of undesirable compounds. The analysis of non-derivatized sugars and sugar alcohols has been effectively conducted through the utilization of gas chromatography/mass spectrometry (GC/MS) with the application of atmospheric pressure chemical ionization (APCI) in negative ion mode.
- Gas chromatography (GC) is a commonly employed technique in forensic science for the purpose of analyzing bodily fluids to identify the presence of illicit substances, examining fibers and blood samples obtained from crime scenes, and detecting traces of explosive residues.
References
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumentation_and_Analysis/Chromatography/V._Chromatography/D._Gas-Liquid_Chromatography
- https://www.broadlearnings.com/courses/gas-liquid-chromatography-glc/
- http://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/S000002BI/P001354/M021511/ET/1501756569ET.pdf
- https://www.brainkart.com/article/Gas-Liquid-Chromatography-%28GLC%29–Instrumentation_30940/
