Gadolinium is a chemical element with an atomic number of 64 and is represented by the symbol ‘Gd’ in the periodic table. It is hard and silvery in appearance and classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. Gadolinium, similar to numerous rare-earth metals, predominantly exhibits the oxidation state of +3 in the majority of its compounds. Nevertheless, gadolinium has been observed to occur in the 0, +1, and +2 oxidation states on infrequent occurrences.
Gadolinium is commonly present in minerals such as monazite and bastnäsite. The presence of the metal in its natural state is hindered by its inherent reactivity. In contrast, gadolinite exhibits a minimal presence of this particular element. The Earth’s crust is estimated to contain approximately 6.2 mg/kg(milligrams per kilogram).
Interesting Science Videos
History of Gadolinium
- In 1880, the esteemed Swiss chemist Jean-Charles Galissard de Marignac made a significant discovery. Through his meticulous observation of spectroscopic lines, he identified the presence of gadolinium, a rare earth element. The observation was made in samples of didymium and gadolinite.
- In 1886, the French chemist Paul Émile Lecoq de Boisbaudran successfully isolated gadolinia, which is the oxide of Gadolinium, from Mosander’s yttria. The isolation of the element occurred relatively recently.
- The element gadolinium, similar to the mineral gadolinite, derives its name from the renowned Finnish chemist and geologist Johan Gadolin.
Occurrence of Gadolinium
- The Earth’s crust is believed to possess an estimated concentration of approximately 6.2 milligrams per kilogram (mg/kg) of gadolinium.
- Gadolinium is not naturally occurring in its elemental form but instead occurs frequently within various minerals, predominantly monazite, and bastnaesite.
- In the commercial setting, the isolation of gadolinium is achieved through the utilization of ion exchange and solvent extraction techniques. The production of metal can be achieved through the process of reducing anhydrous gadolinium fluoride using calcium metal.
- Gadolinium demonstrates a comprehensive collection of 27 isotopes, encompassing mass numbers ranging from 137Gd to 164Gd, for which the corresponding half-lives have been established. The naturally occurring gadolinium is comprised of a mixture of seven isotopes.
Isotopes of Gadolinium
The naturally occurring gadolinium is comprised of a mixture of seven isotopes: 152Gd, 154Gd, 155Gd, 156Gd, 157Gd, 158Gd, and 160Gd.
Naturally Occurring Isotopes of Gadolinium
| Isotopes | Natural Abundance (atom %) |
|---|---|
| 152Gd | 0.2 |
| 154Gd | 2.2 |
| 155Gd | 14.8 |
| 156Gd | 20.5 |
| 157Gd | 15.7 |
| 158Gd | 24.8 |
| 160Gd | 21.9 |
Elemental Properties of Gadolinium
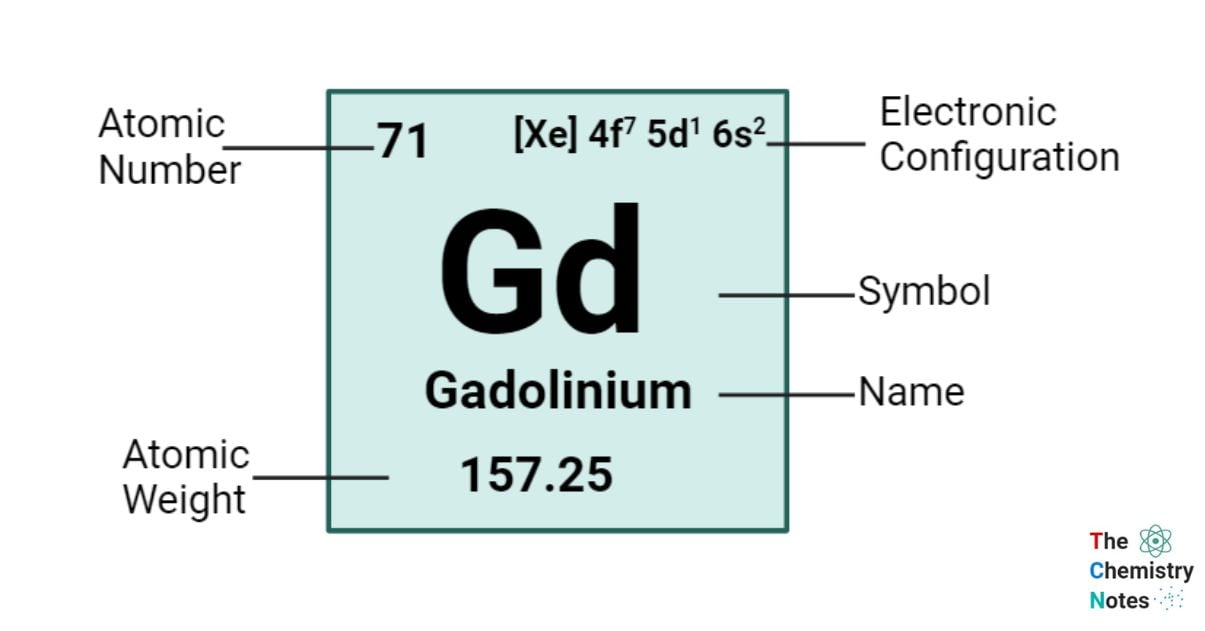
| Electronic Configuration | [Xe] 4f7 5d1 6s2 |
| Atomic Number | 64 |
| Atomic Weight | 157.25 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Lanthanides, 6, f-block |
| Density | 7.895 g.cm -3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 25, 9, 2 |
| Electrons | 64 |
| Protons | 64 |
| Neutrons in the most abundant isotope | 94 |
Physical Properties of Gadolinium
- Gadolinium has an atomic number of 64 and is a silvery-white rare earth metal. It has a melting point of 1312°C (2394°F) and a boiling point of 3273°C (5923°F).
- Gd has a solid phase density of 7.90 g/cm3 and a liquid or molten phase density of 7.4 g/cm3.
- It is malleable which means it can be easily beaten into thin sheets without any cleavage.
- It is ductile which means it is possible to draw thin wires from it without breaking.
- Gadolinium displays ferromagnetic properties at temperatures below 20°C but demonstrates strong paramagnetic behavior when the temperature surpasses this threshold.
- At room temperature, gadolinium exhibits crystallization in a hexagonally closed-packed structure. The transformation occurs at a temperature of 1235°C, resulting in a shift to a body-centered-cubic structure.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1585 K (1312 °C, 2394 °F) |
| Boiling point | 3273 K (3000 °C, 5432 °F) |
| Density | 7.90 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.1 (Pauling Scale) |
Chemical Properties of Gadolinium
- Gadolinium demonstrates a limited degree of reactivity when exposed to dry air. Nevertheless, upon exposure to humid air, the substance experiences a process known as tarnishing, which leads to the creation of a powdery white oxide. Regrettably, the present oxide layer fails to offer adequate protection against subsequent oxidation.
- Gadolinium exhibits a significant degree of electropositivity. The reaction exhibits a sluggish rate when exposed to cold water, while its rate is significantly accelerated when subjected to hot water.
- Because of its high reactivity, gadolinium readily forms compounds of the element Gd (III) when combined with other elements.
- When it is found in compounds, gadolinium almost always resides in the trivalent form, denoted by the symbol Gd3+.
- The interaction of the metal gadolinium with water is extremely sluggish, yet it is possible for it to be dissolved in dilute acid. It leads to the creation of salts that are colorless in appearance.
- Gadolinium demonstrates reactivity with all halogens at a temperature of 200°C, leading to the creation of distinct trihalides corresponding to each halogen.
Chemical Reaction of Gadolinium
- The Reaction of Gadolinium With Water
Gadolinium exhibits a sluggish reaction when exposed to cold water, while it displays a rapid reaction when in contact with hot water. This reaction leads to the formation of gadolinium hydroxide (Gd(OH)3) and the liberation of hydrogen gas (H2).
2 Gd (s) + 6 H2O (g) → 2 Gd(OH)3 (aq) + 3 H2 (g)
- The Reaction of Gadolinium With Air
Gadolinium exhibits a slow reaction with oxygen at room temperature, and it readily undergoes combustion to produce gadolinium (III) oxide, also known as Gd2O3.
4 Gd (s) + 3 O2 (g) → 2 Gd2O3 (s)
- The Reaction of Gadolinium With Halogens
The element gadolinium exhibits a propensity to engage in chemical reactions with various halogens, resulting in the formation of gadolinium (III) halides.
The chemical reaction between gadolinium metal and fluorine gas (F2) results in the formation of gadolinium (III) fluoride, denoted as GdF3.
2 Gd (s) + 3 F2 (g) → 2 GdF3 (s) [white]
The chemical reaction between gadolinium metal and chlorine gas (Cl2) results in the formation of gadolinium (III) chloride, denoted as GdCl3.
2 Gd (s) + 3 Cl2 (g) → 2 GdCl3 (s) [white]
The chemical reaction between gadolinium metal and bromine (Br2) results in the formation of gadolinium (III) bromide, denoted as GdBr3.
2 Gd (s) + 3 Br2 (g) → 2 GdBr3 (s) [white]
The chemical reaction between gadolinium metal and iodine represented as I2, results in the formation of gadolinium (III) iodide, denoted as GdI3.
2 Gd (s) + 3 I2 (g) → 2 GdI3 (s) [yellow]
- The Reaction of Gadolinium With Acid
Gadolinium metal exhibits a high degree of solubility in dilute sulfuric acid, resulting in the formation of colorless Gd (III) ions and the liberation of hydrogen gas, H2.
2 Gd (s) + 3 H2SO4 (aq) → 2 Gd3+ (aq) + 3 SO42− (aq) + 3 H2 (g)
Uses of Gadolinium
Numerous significant applications exist for gadolinium and its compounds some of which are discussed here:
Used In Alloys
Gadolinium exhibits atypical metallurgical characteristics. The inclusion of a mere 1% of gadolinium in iron, chromium, and their respective alloys significantly enhances their malleability, as well as their ability to withstand elevated temperatures and resist oxidation. Gadolinium is employed in iron and chromium alloys to enhance their capacity to withstand elevated temperatures and resist oxidation.
Used In Nuclear Reactors
Gadolinium is employed as a burnable toxin in nuclear marine systems for propulsion. The presence of gadolinium in the system leads to a deceleration of the initial reaction rate. However, as the gadolinium undergoes decay, additional neutron poisons gradually accumulate, thereby facilitating the sustained operation of the reactor cores over an extended period. In certain nuclear reactors, specifically those of the CANDU variety, gadolinium is employed as an additional safety mechanism for emergency shutdown purposes.
Used For Imaging
Due to their paramagnetic properties, organic gadolinium complexes and gadolinium compounds are used as radiocontrast agents in medical magnetic resonance imaging (MRI) that is given through an IV (intravenous). These solutions enhance the quality of images, allowing for improved visualization during the imaging process. In addition to its use in magnetic resonance imaging (MRI), gadolinium (Gd) is also employed in various other imaging techniques. Gadolinium is present in the phosphor layer that is suspended within a polymer matrix in X-ray detectors. The phosphor layer is made of terbium-doped gadolinium oxysulfide (Gd2O2S: Tb), which changes the X-rays coming from the source into visible light.
Used In Magnetic Refrigeration
Gadolinium, silicon, and germanium alloys show a strong magnetocaloric effect after being melted using an arc at room temperature. This effect causes a temperature increase when exposed to a magnetic field and a decrease when removed from it. This characteristic makes them useful for room-temperature magnetic refrigeration.
Miscellaneous Applications
- Gadolinium is employed as a dopant in the production of fuel cells, similar to the utilization of cerium oxide, which is considered an optimal and cost-efficient approach.
- Color television sets favor gadolinium in the form of phosphorus, and microwave-related appliances also use it.
- In order to replicate the appearance of diamond gemstones and jewelry, craftsmen commonly employ a material known as Gadolinium Gallium Garnet (Gd3Ga5O12).
Health Effects of Gadolinium
- Like other elements in the lanthanide series, gadolinium has the capacity to create compounds that demonstrate levels of toxicity ranging from low to moderate.
- There have been observations indicating that Gadolinium salts can cause irritation in the skin and eyes. Additionally, there is a suspected link between Gadolinium salts and the development of tumors.
- The current understanding of gadolinium toxicity is still limited, with only a limited amount of detailed investigation conducted thus far.
Environmental Effects of Gadolinium
Currently, there is a lack of knowledge regarding the potential environmental impacts of gadolinium. It appears that animals and plants are not significantly impacted.
References
- https://www.rsc.org/periodic-table/element/64/gadolinium
- https://www.sciencedirect.com/topics/chemistry/gadolinium
- https://en.wikipedia.org/wiki/Gadolinium#:~:text=Gadolinium%20is%20a%20chemical%20element,a%20ductile%20rare%2Dearth%20element.
