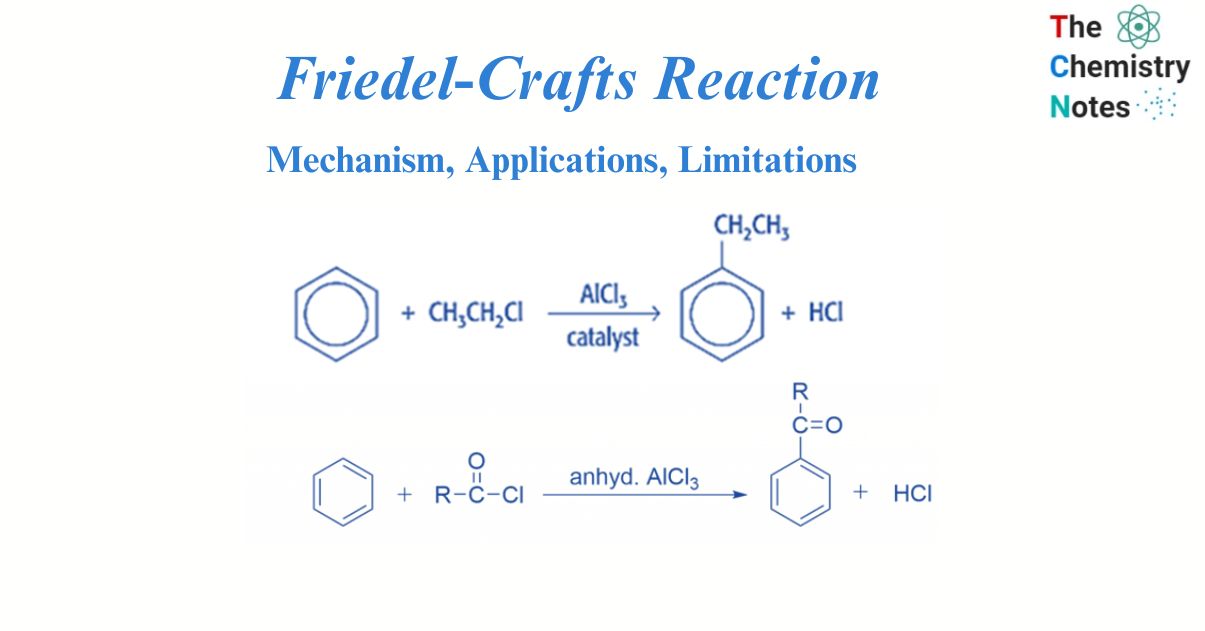
Friedel-Crafts reaction is an electrophilic aromatic substitution reaction. This well-known reaction was invented by two scientists, French Charles Friedel and American James Crafts. In the Friedel-Crafts reaction, the aromatic molecule undergoes an electrophilic substitution. In this reaction process, electrophile replaces the hydrogen atom in the benzene, in the presence of a Lewis acid such as anhydrous aluminum chloride.
There are two types of Friedel crafts reactions i.e., alkylation reactions and acylation reactions. Both of them occur via electrophilic substitution reaction. Electrophilic aromatic substitution reactions occur in benzene. An organic reaction in which an atom connected to an aromatic system, usually hydrogen, is replaced by an electrophile is known as electrophilic aromatic substitution.
Interesting Science Videos
What is Friedel-Crafts alkylation?
Friedel-Crafts alkylation is the substitution of an aromatic proton by an alkyl group. This is accomplished through an electrophilic attack on the aromatic ring using a carbocation. The Friedel-Crafts alkylation reaction uses alkyl halides as reactants to produce alkylbenzenes.
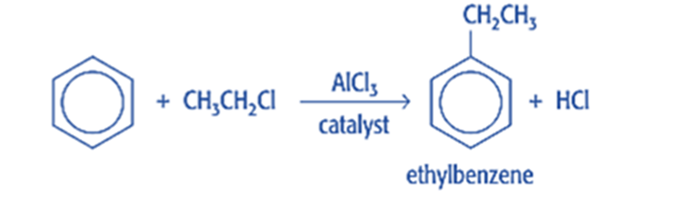
Mechanism of Friedel-Crafts alkylation reaction
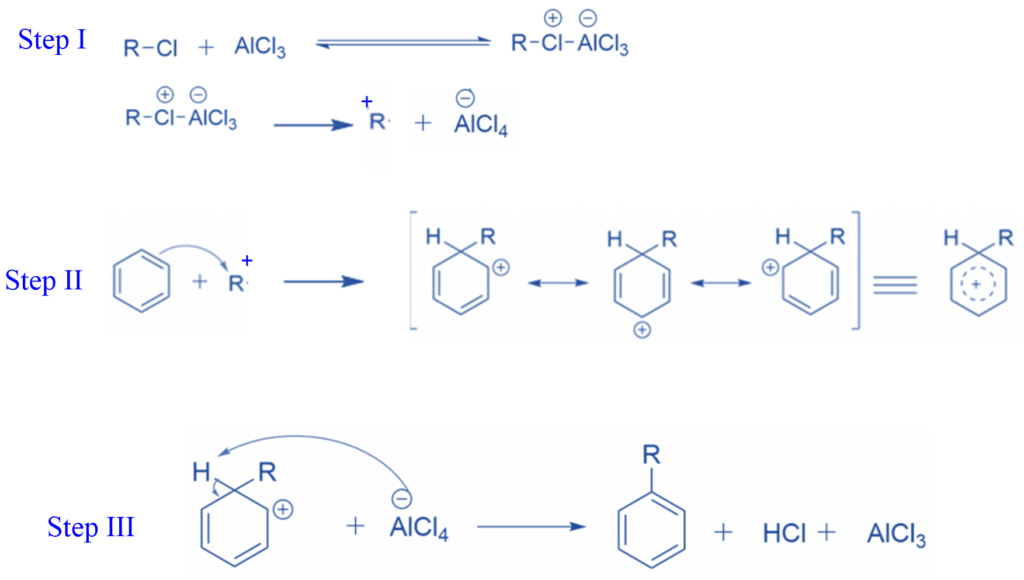
Step I: The halogen atom of the alkyl halide transfers a lone pair to a vacant orbital on the Al atom of AlCl3 to form electrophile, in the first step of the Friedel-Crafts alkylation process.
Step II: The electrons of the benzene ring attack the electrophile.
Step III: The aromaticity of the benzene ring is disturbed when the carbon-carbon bond is formed using benzene -electrons. Because aromatic rings are quite energetically stable, there is a significant drive for the benzene ring to restore aromaticity. To achieve this, the carbon atom that is attached to the new alkyl substituent on the benzene ring must lose its hydrogen atom. A base can achieve this; in this reaction, one of the chloride ions coordinated with the Al in AlCl4– can be used.
What is Friedel-Craft’s acylation reaction?
The Friedel-Crafts acylation reaction includes the addition of an acyl group to an aromatic ring. In this reaction, Benzene combines with acid chloride in the presence of a Lewis acid catalyst, such as AlCl3, to produce ketone.

Mechanism of Friedel-Crafts acylation reaction
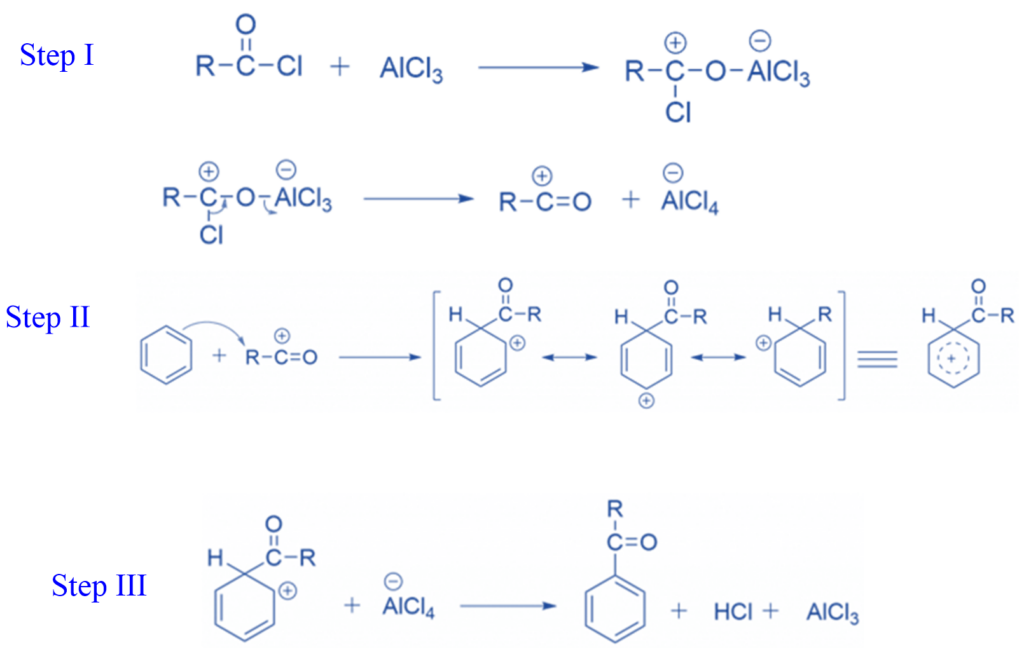
Step I: Chlorine develops a Lewis acid-base complex with aluminium chloride in the first step, which then functions as a leaving group. This results in the formation of a resonance-stabilized acyl cation.
Step II: The acyl cation reacts with benzene during acylation. A nucleophilic benzene bond forms a new C-C bond with the electrophilic acyl carbon.
Step III: AlCl4– abstracts the hydrogen to regenerate the aromatic double bond. This regenerates the aluminium chloride catalyst while producing HCl.
Preference of Friedel-Crafts acylation over alkylation
In the Friedel craft reaction, acylation is favored over alkylation. This is further electrophilic substitution is not allowed in the acylation because the acyl group, an electron-withdrawing group, lowers the electron density. Because the carbon chain of acyl halide does not rearrange, there is no likelihood of isomeric ketone molecules forming.
In contrast, in alkylation, the methyl group acts as an electron donor, increasing the electron density and electrophilic reactivity of the substrate. As a result, poly alkylation occurs rather than mono alkylation. This provides a “workaround” to produce compounds that would otherwise be challenging to obtain through Friedel-Crafts alkylation because of carbocation rearrangements.
Application of Friedel-Crafts reactions
- During the organic reaction process, this reaction is commonly used to form C-C bonds.
- One of the most important applications of this reaction is the generation of dyes, such as xanthene dyes.
- It is also employed in the Haworth reactions.
- It is employed in the synthesis of aromatic compounds with functional groups such as aldehydes and ketones.
- Friedel-Crafts alkylation is widely used in the complete synthesis of natural products and complex compounds, which are then used for a variety of biological purposes.
- Friedel-Crafts reaction is utilized to create a wide range of aromatic compounds with various substituents.
- Friedel-Crafts reaction is connected to several well-known named reactions, such as the Clemmensen Reduction.
- Friedel-Crafts reaction is utilized in the manufacture of Triarylmethane (CH(C6H5)3) dyes.
- Friedel-Crafts reaction process is employed in the detection of aromatic substances.
Limitation of Friedel-Crafts reactions
- The Friedel-Crafts technique only yields ketone molecules. Under specific conditions (CO), formyl chloride degrades into HCl and CO2.
- Due to their low reactivity, mono halobenzenes do not respond to or participate in the Friedel-Crafts acylation process.
- Carbocations are quickly rearranged to more stable carbocations, and the carbocation formed during Friedel-Crafts alkylation may undergo rearrangement prior to the attack on the aromatic ring. This results in the creation of a product, which is replaced by rearranged carbocation.
- Because the alkyl group activates the ring, it is obvious that the product of Friedel-Crafts alkylation will be more reactive and capable of additional alkylation. As a result, we get both monoalkylated and polyalkylated compounds. However, polyalkylation can be reduced by using an appropriate amount of aromatic substance.
- Friedel-Crafts reactions cannot be performed when the aromatic ring has an NH2, NHR, or NR2 substituent. The amines’ lone pair electrons react with the Lewis acid AlCl3. This places a positive charge close to the benzene ring, which is so powerfully activating that the Friedel-Crafts reaction is prevented.
- Isomerization and disproportionation can occur in the presence of excess catalysts and at high temperatures.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
