Formal charge calculation is significant since it is used to determine the lowest energy configuration among the different potential Lewis structures of a molecule. An atom in a molecule is given a formal charge if all of the electrons in the chemical bonds are distributed evenly among the atoms. This assumption does not account for the atoms’ differences in electronegativity. The total of all the atoms’ formal charges equals the actual charge of the compound. The compound has a net formal charge of zero if it is neutral.
Interesting Science Videos
Formal Charge Calculation
The formal charge of a molecule may be computed using following method based on its Lewis structure representation.
The formal charge of an atom in a molecule is given by the following formula.
Formal Charge = Valence electrons of a free atom – Non-bonding electrons (lone pairs) – ½(Bonding electrons)
When calculating the formal charge on molecules’ atoms, there are a few criteria that need to be considered.
- For a molecule to be considered neutral, its formal charge must always be 0.
- Whether an ion is an anion or a cation, its formal charge must match the charge on that ion.
Examples of Formal Charge Calculation
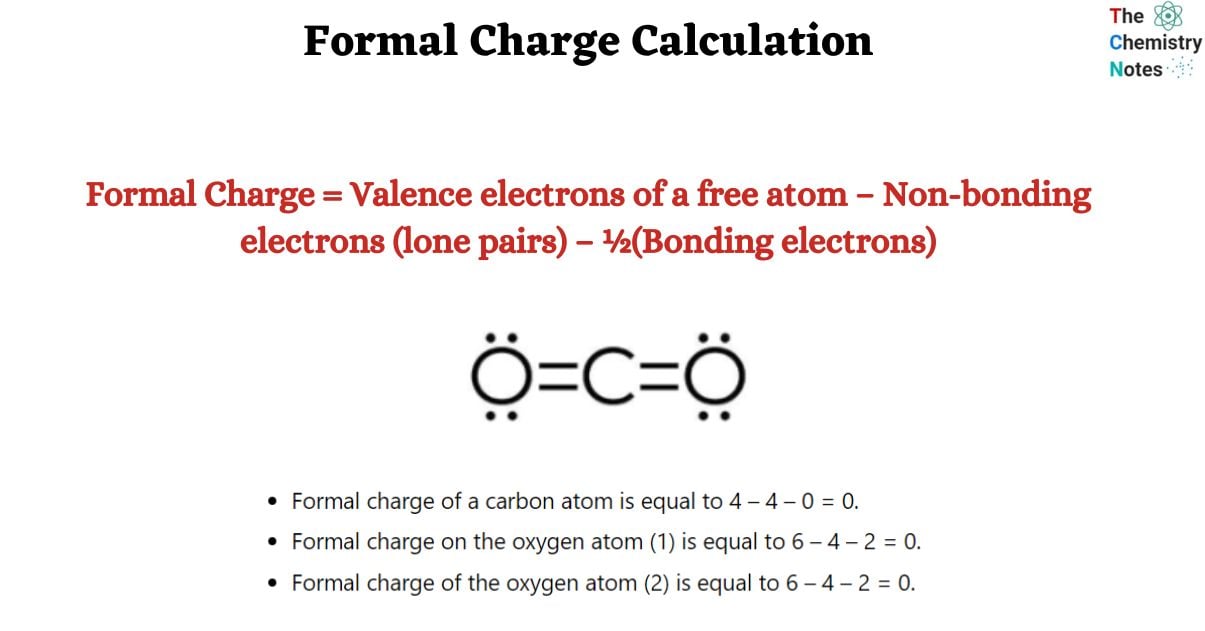
Formal Charge of Carbon dioxide
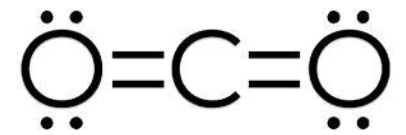
Formal charge = V (free atom) – Non-BE (lone pairs) – ½ BE (bond pairs)
- Formal charge of a carbon atom is equal to 4 – 4 – 0 = 0.
- Formal charge on the oxygen atom (1) is equal to 6 – 4 – 2 = 0.
- Formal charge of the oxygen atom (2) is equal to 6 – 4 – 2 = 0.
Thus, carbon dioxide has no formal charge.
Formal Charge of Sulfur dioxide
Formal charge = V (free atom) – Non-BE (lone pairs) – ½ BE (bond pairs)
- Formal charge on an atom of sulfur is equal to 6 – 2 – 4 = 0.
- Formal charge on oxygen atom (1) is equal to 6 – 4 – 2 = 0.
- Formal charge on oxygen atom (2) is equal to 6 – 4 – 2 = 0.
As a result, the total formal charge on sulfur dioxide is zero.
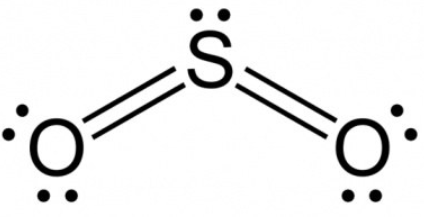
Formal charge on ammonium ion
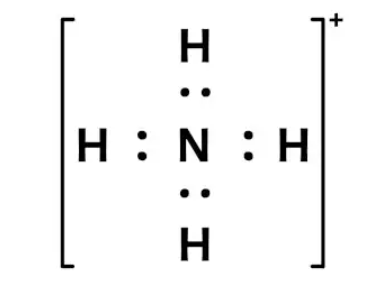
Formal charge = V (free atom) – Non-BE (lone pairs) – ½ BE (bond pairs)
- On a nitrogen atom, the formal charge is 5 – 0 – 4 = +1.
- Formal charge on the hydrogen atom (1) is equal to 1 – 0 – 1 = 0.
- Formal charge on the hydrogen atom (2) is equal to 1 – 0 – 1 = 0.
- Formal charge on hydrogen atom (3) is equal to 1 – 0 – 1 = 0
- Formal charge on hydrogen atom (4) is equal to 1 – 0 – 1 = 0
Thus, the ammonium ion’s total formal charge is [+1 + 0 + 0 + 0 + 0 ]. = 1
Significance of Formal Charge
- A molecule can exist in a variety of structures. The energy content is the factor that allows it to exist in a free condition. The structure with the lowest energy is the most likely to exist. Because the energy of molecules cannot be directly measured, there is a concept known as formal charge that may predict if a certain structure has a high or low energy relative to others.
- Accordingly, formal charge estimates the relative stability of potential structures based on their formal charge values rather than the precise quantity of energy of a molecule.
- The formal charge is utilized for predicting the potential arrangement of molecules. This is important due to the fact that correctly predicting the most stable configuration of a product in a reaction allows a chemist to change the production rates and emphasis the output product.
- Furthermore, it is the formal charge that leads to advanced concepts such as valence bond theory (VBT), molecular orbital theory (MOT), and so on.
Video on Formal Charge Calculation
References
- https://collegedunia.com/exams/formal-charge-chemistry-articleid-6069
- https://psiberg.com/formal-charge/
- https://www.studysmarter.co.uk/explanations/chemistry/ionic-and-molecular-compounds/formal-charge/
- https://www.masterorganicchemistry.com/2010/09/24/how-to-calculate-formal-charge/
- https://byjus.com/chemistry/is-formal-charge-a-fake-charge/
- https://www.chemistrylearner.com/formal-charge.html#google_vignette

