Fluorescence Spectroscopy is a set of techniques that deals with the measurement of fluorescence emitted by substances when exposed to ultraviolet, visible, or other electromagnetic radiation.
It has wide application in chemical and biological sciences as it can be used to analyze a biological system, by studying its interactions with fluorescent probe molecules. When scientists first started looking at fluorescence in the early 20th century, it sparked the development of fluorescence spectrophotometry. The modern method of Fluorescence Spectroscopy has its roots in the 1920s and 1930s when scientists like George Van Nevel and Robert Boyle began developing techniques for quantifying fluorescence.

In many fields, including chemistry, biology, and medicine, fluorescence spectrophotometry is a crucial tool. It is a tool used in chemistry for the study of molecular properties and the determination of previously unknown chemicals. It is employed in the study of biomolecular structure and function. In medicine, it is employed for the purposes of disease diagnosis and the study of drug-biological interaction.
Interesting Science Videos
Fluorescence spectrometry: Definition
Fluorescence spectrometry is a fast, simple, and low-cost method for estimating the concentration of an analyte in solution based on the analyte’s fluorescent properties. Simple approaches can be used to determine the concentration of the analytes when the type of substance being analyzed (the “analyte”) is known. Typically, chemicals in solution are the ones being measured by fluorescence.
In fluorescence spectroscopy, a cuvette holding a solution is passed through a beam of light having a wavelength between 180 and 800 nm. After that, we take an off-angle reading of the sample’s luminescence. Both the excitation spectrum (the light received by the sample) and the emission spectrum (the light produced from the sample) can be measured in fluorescence spectrometry. There is a linear correlation between analyte concentration and emission strength.
The Fluorescence Spectroscopy Principle
- When a molecule absorbs energy at a wavelength where it has a dipole moment of transition, it emits electromagnetic radiation in the form of fluorescence. After being propelled to an excited singlet state by the molecule’s provided excitation energy in its ground state, photons eventually decay to the state’s lowest vibrational energy level. Photons are created as the molecule’s energy levels return to their ground state.
- Internal conversion takes place whenever there is a tiny energy gap between two electronic states, allowing electrons to transition from a higher to a lower energy level. In this region, the vibrational modes of the electronic state get the energy. Since thermal energy is what drives vibrational processes, raising the temperature dampens the brightness of fluorescence. In the process of external conversion, energy is dissipated due to collisional quenching with solute molecules in the fluorophore’s environment.
- When the energy levels of the excited singlet and triplet states overlap, electrons can cross over from the ground state to the first excited triplet state, a phenomenon known as intersystem crossover.
- Phosphorescence is the process through which electrons transition back to their ground state and release photons. Phosphorescence peaks are at longer wavelengths than fluorescence peaks due to the lower energy of the triplet state compared to the singlet state.
- Phosphorescence lasts longer than fluorescence ((~10-4 – 10-2 seconds vs. ~10-9 – 10-6 seconds) because these transitions are also forbidden. Molecular collisions, migration of the solvent, and oxygen quenching all contribute to thermal deactivation as lifetimes increase. Therefore, samples need to be chilled to liquid nitrogen temperature in order to detect phosphorescence.
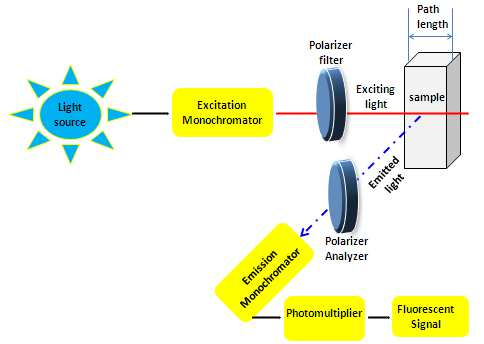
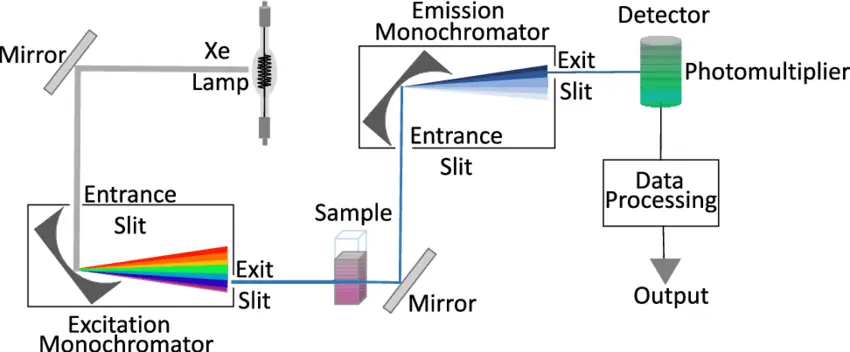
Instrumentation of Fluorescence Spectrophotometry
A light source, sample holder, and detector are the three mainstays of any fluorescence equipment. The detector signal also needs to be precisely adjustable and presented for analytical purposes, and the wavelength of the incident radiation should be selectable.
Fluorescence Spectroscopy Light Sources
Depending on the technique, a number of different light sources can be used for fluorescence spectroscopy.
- It is possible to create UV light with relatively simple light sources, such as some gas discharge lamps (such as mercury vapor lamps), by using either their continuous emission or their pulsed emission. As a result, a bandpass optical filter is commonly employed for frequency band reduction. Excitation monochromator sources that sweep through a range are also used in the measurements.
- Even light-emitting diodes (LEDs) can do the job under some conditions. This allows for low-cost and compact options, which are especially useful for mobile gadgets.
- Lasers of various varieties are powerful excitation sources because they can stimulate a wide range of wavelengths. This includes ultraviolet, which is often produced by frequency doubling in a nonlinear crystal. Light with a small optical bandwidth (linewidth) could be useful for excitation. In addition, nanosecond, picosecond, or femtosecond-long pulses can be easily generated with pulsed lasers.
- Tunable lasers, OPOs, or a broadband source coupled with an excitation monochromator are all viable options for producing a wide range of excitation wavelengths. The excitation intensities available in the latter case are, of course, much lower than those provided by lasers.
Photodetectors
- Various photodetectors may be used, depending on the situation. Devices with continuous-wave excitation and a scanning monochromator only need a photodetector with high sensitivity in the appropriate spectral area, like a photodiode. An even more sensitive detector is needed besides a dual monochromator for maximum suppression of background light.
- Another alternative is to use a spectrograph equipped with a diffraction grating in conjunction with a linear photodiode array or some other type of photodetector that has a good spatial resolution. Both spatial and spectral resolutions of one dimension are feasible with a two-dimensional sensor like an image sensor.
- The measurement of fluorescence lifetimes calls for both high sensitivity and a fast response time (high bandwidth). This narrows down the pool of viable detectors to ones like photomultipliers and avalanche photodiodes. There are also gadgets that feature a microchannel plate.
Sample holders
- The majority of fluorescence measurements are taken in a cuvette or flow cell and performed on a solution. Cuvettes are light-tight containers that can be either round, square or the increasingly rare rectangular shape.
- Square cuvettes or cells are the most precise because path length and parallelism may be more easily maintained during manufacture. It’s more practical and economical to use a round cuvette for many common tasks. The cuvette is pointed directly into the light source.
- The fluorescence can be gathered from the front surface of the cell, perpendicular to the incident beam, or in the direction of the beam. The technique of collection can be tailored to the characteristics of the sample in some instruments. A highly dilute solution will emit light uniformly throughout the path of the incident beam.
- Light scattered by the solution and cell is reduced to a minimum with the right-angled collecting method. This situation is used in analytical measurements. The device only collects a negligible amount of fluorescence at each location along the light path, and this is what is sent to the detector.
- This means that a lower volume of solution can be contained in a microcell whose dimensions better match the optical requirements of the instrument without compromising the recorded intensity of the fluorescence signal.
- The fluorescence emission becomes distorted as the solution absorbs more, eventually penetrating only the front surface of the cuvette. Measurements may be taken thanks to light collected from the front cover, although the amount of light scattered by the cuvette wall is significant.
- Emission (perhaps distorted) from a fluorescent sample will always be visible in the front surface collection, while in 90° collection, the fluorescence gradually declines with increasing solution absorbance. A sample’s fluorescence can be destroyed at very high concentrations. The concentration of a completely unknown solution must be adjusted to 0.1 A and the absorbance must be measured.
Applications of fluorescence Spectroscopy
Almost any field you can think of can benefit from fluorescence spectroscopy. It is difficult to imagine the concept of modern chemical and biological research without this method.
Bioscience
- One of the most common uses of fluorescence spectroscopy in biosciences is the highly accurate measurement of molecules like DNA and RNA. The concentration of a DNA sample is measured after an extrinsic fluorophore (often ethidium bromide) has been added and the sample has been fed into a fluorescence spectrometer.
- Today’s technology also allows for single-molecule real-time (SMRT) DNA sequencing. It is expected to play a pivotal role in the next generation of the genetic diagnostic revolution due to its ability to synthesize long-read single molecules with excellent precision.
- Fluorescence spectroscopy in the industry is widely utilized for contamination assessment because it is a quick and noninvasive method. Hydraulic fracturing for gas exploration can contaminate the groundwater with organic chemicals; this technology has been utilized to detect these molecules.
Chemical
- The production of nanoparticles with potential medicinal applications, such as medication delivery, is one example of an important chemical use of fluorescence spectroscopy.
- Proteins and other biomolecules (the “protein corona”) bind to and coat nanoparticles when they come into contact with bodily fluids. Nanoparticle safety in vivo is affected by its interactions with the protein corona.
- Researching these interactions is essential for comprehending. Techniques like time-resolved fluorescence quenching and fluorescence correlation spectroscopy are utilized for this purpose.
- The approach also has considerable potential in the field of environmental surveillance. One such case is the purification of water close to landfills.
- Landfill leachates are produced when liquid waste biodegrades in a landfill and rainwater percolates through the garbage. The chemical cocktail found in leachate can do serious damage to ecosystems.
Pharmaceutical
- The pharmaceutical industry also makes use of spectrofluorometric methods for medication analysis. An examination of co-formulated tablets used to treat cholesterol is one such instance.
- Atoreza, a pill containing Ezetimibe and Atorvastatin calcium, may be analyzed quickly, easily, and precisely with synchronous fluorescence spectroscopy.
Agricultural
- Spectroscopic techniques find extensive use in agriculture, for instance in the identification of crop varieties.
- The use of total luminescence spectroscopy to differentiate between identical varieties of tea provides a fast, cheap, and objective alternative to using professional tea tasters
Factors Affecting fluorescent spectroscopy
- Rigid fluorophores, which are less likely to undergo a transition to a triplet state, are preferred for use in fluorescence spectroscopy. Phenolphthalein, in contrast to fluorescein and eosin, which have rigid structures and produce bright light, is soft and non-fluorescent.
- The polarity of the solvent is another factor in determining the intensity of fluorescence. The structures’ fluorescence can be dampened by the presence of heavy atoms in the solvent.
- Oxygen dissolved in the solvent is another factor that might dampen the brightness of fluorescent emission. To do this, fluorophore undergoes photochemical oxidation. Fluorescence can be quenched by oxygen due to its paramagnetic properties.
- A substance’s fluorescence may change depending on its pH. Aniline is a prototypical example of a substance that changes its ionic charge depending on the pH level. In both cases, fluorescence is destroyed.
- The reduction in fluorescence intensity is known as “quenching.” This could be because the solution absorbs the fluorescence or because the fluorescent ingredient is absorbed. The term “self-quenching” describes this phenomenon.
- To absorb UV/vis light, molecules must be unsaturated (have electrons), a process known as conjugation. Fluorescence cannot take place until some radiation is absorbed.
- Differences in fluorescence emission between flexible and rigid structures can be attributed to their respective rigidities.
- Fluorescence is improved by the presence of electron-donating groups like amino and hydroxyl. Fluorescence is dampened by the presence of electron-withdrawing compounds like nitro and carboxyl. Groups like SO3H and NH4+ have no effect on fluorescence intensity.
- The effect of temperature is that as it rises, molecular collisions rise and fluorescence intensity falls, while as it falls, collisions reduce and fluorescence intensity rises.
- Increasing viscosity improves fluorescence intensity because fewer molecules collide with one another, while lowering viscosity decreases fluorescence intensity because more molecules collide with one another.
Advantages of Fluorescence spectroscopy
Compared to other spectroscopic methods, fluorescence spectroscopy has many benefits.
- Because of its high sensitivity, fluorescence spectroscopy is ideally suited for identifying minute quantities of contaminants like biological molecules, pollutants, and more.
- Using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small groups of molecules.
- Fluorescence spectroscopy is useful for detecting faint fluorescence signals because of its low background noise.
- Using numerous fluorescent labels, fluorescence spectroscopy can detect multiple molecules in parallel, a process known as multiplexing.
- There is no sample destruction or modification during fluorescence spectroscopy analysis.
- Using fluorescent labels that have been directed to specific cells or tissues, fluorescence spectroscopy can be used in vivo to image biomolecules in motion.
- Because of its low price and ease of use, fluorescence spectroscopy is used by a wide range of scientists.
- Fluorescence spectroscopy has several potential uses in fields as disparate as chemistry, biology, medicine, environmental monitoring, and industrial analysis, demonstrating its versatility.
Fluorescence spectroscopy’s drawbacks
There are benefits to using fluorescence spectroscopy, but the technique is not without its drawbacks. Some major drawbacks include:
- When fluorescent molecules are exposed to light, they lose their fluorescence, a process known as photobleaching. This can happen in fluorescence spectroscopy, causing the fluorescence signal to weaken with time.
- Reduced fluorescence is caused by quenching, in which one molecule blocks the light from another.
- Interference from other sources: Scatter, absorption, and autofluorescence can all alter the results of a fluorescence spectroscopy experiment, making it more difficult to understand the data.
- Fluorescence spectroscopy is not well suited for examining deep tissue samples or samples that are not transparent to the excitation light due to its restricted penetration depth.
- Because the fluorescence signal requires fluorescent molecules to function, it cannot be used on samples that do not contain these substances.
- Not applicable to all molecular species due to its limited detection range; fluorescence spectroscopy can only be used to detect fluorescing molecules.
- Due to its limited dynamic range, fluorescence spectroscopy is only able to detect concentrations within a narrow range.
- Costly reagents are needed for several fluorescence spectroscopy applications, particularly fluorescent labels.
- Careful buffering is essential since it can have a significant impact on fluorescence intensity.
- When exposed to ultraviolet radiation, the fluorescent molecule may undergo photochemical modifications or even be destroyed.
- Dissolved oxygen may contribute to more photochemical damage.
- Iodide and nitrogen oxides, even in small concentrations, are powerful quenchers and can cause interference.
- Due to its poor accuracy for large volumes, the method is unfit for identifying the primary components of a sample.
- This method’s utility is restricted by the fact that not all compounds or elements emit light.
- While fluorescence spectroscopy has many applications, it is important to be aware of its limitations and drawbacks before beginning an experiment or interpreting the results.
References
- Albani, J., 2007. Principles and applications of fluorescence spectroscopy. Oxford; Ames, Iowa: Blackwell Science.
- Ardui, S., Ameur, A., Vermeesch, J.R. and Hestand, M.S., 2018. Single-molecule real-time (SMRT) sequencing comes of age: applications and utilities for medical diagnostics. Nucleic acids research, 46(5), pp.2159-2168.
- Sobarwiki, 2013. Simplified layout of a fluorescence spectrophotometer setup.
- https://microbiologynote.com/fluorescence-spectrophotometry-definition-principle-parts-advantages-uses/#google_vignette
- https://www.labcompare.com/Spectroscopy/411-Fluorescence-Spectrometer/
