Flame Photometry is a type of atomic spectroscopy and is used in chemical analysis to quantify the concentration of metal ions such as sodium, potassium, lithium, calcium, and so on.
The species (metal ions) employed in the spectrum in flame photometry are in the form of atoms. Flame atomic emission spectrometry (FAES) has been proposed by the International Union of Pure and Applied Chemistry (IUPAC) Committee on Spectroscopic Nomenclature. The species of alkali metals (Group 1) and alkaline earth metals (Group II) metals are dissociated as a result of the thermal energy delivered by the flame source. Some of the atoms are stimulated to a higher energy level as a result of this thermal excitation, where they are not stable.
The absorbance of light which cause electron excitation can be assessed through direct absorption techniques. After the subsequent loss of energy, excited atoms move to the low energy ground state and emit certain radiations that can be seen in the visible region of the spectrum.
Metal ions, which are employed in the spectrum, are in the form of atoms. Flame photometry determines the concentration of metal ions based on the intensity of the light emitted when alkali metals or alkaline earth metals are added to the flame. As a result, flame emission spectrophotometry is based on the distinctive emission of light by atoms of many metallic elements when given enough energy, such as that supplied by a hot flame. The wavelength used for examining an element is determined by selecting a line with sufficient intensity to offer acceptable sensitivity and freedom from other interfering lines at or near the chosen wavelength.
Interesting Science Videos
History of Flame Photometry
Bowling Barnes, David Richardson, John Berry, and Robert Hood designed a device in the 1980s for detecting low sodium and potassium concentrations in a solution. This apparatus was given the name Flame Photometer. The flame photometer principle is based on measuring the intensity of emitted light when a metal is introduced into the flame. The wavelength of the color indicates the element, and the color of the flame indicates the amount of the element contained in the sample. Earlier, the presence of metal elements in samples aspirated into a flame was detected. The modern analytical Flame Atomic Emission Spectroscopy i.e. Flame Photometry was founded by Lundegardh in 1934.
Principle of Flame Photometry
The flame photometry principle is based on measuring the intensity of the emitted light when a metal is introduced into the flame. The color of the wavelength and its intensity both reveal information about the elements present in the given sample and their relative concentrations, respectively.
When alkali and alkali earth metal compounds are placed into the flame, they break down into atoms. These neutral atoms receive energy from thermal energy and are then stimulated to a higher energy level. However, they are not stable at greater energy levels.
As a result, these atoms, which are unstable at greater levels, return to the ground state. When these atoms return to their ground state, they emit radiation of a specific wavelength, primarily in the visible range.
When a solution containing a significant amount of the metal is injected into the flame, the following events occur in fast succession:
Liquid sample → Formation of droplets → Fine residue → Formation of neutral atoms → Excitation of atoms by thermal energy → Emission of radiation of specific wavelength
Wavelength and intensity of the emitted radiation are measured
The wavelength of the emitted radiation is given by the following equation:
As we know, E2 – E1= hc / λ
Therefore, wavelength λ= hc/ E2-E1
Where,
h= Planks constant
c= velocity of light
E2= energy level at an excited state
E1= energy level at ground state
The intensity of the emission is directly proportional to the number of atoms which return to the ground state from the excited state from the higher levels. The amount of light emitted is proportional to the concentration of the given sample.
The wavelength of the radiation emitted is characteristic of the elements and is used to identify the elements (Qualitative Analysis). There is a specific wavelength for each of the alkali metals and alkaline earth metals which is shown in the table below:
| Element | Wavelength emitted | Flame colour |
| Lithium | 670 nm | Red |
| Sodium | 589 nm | Yellow |
| Potassium | 766 nm | Violet |
| Calcium | 622 nm | Orange |
| Barium | 554 nm | Lime green |
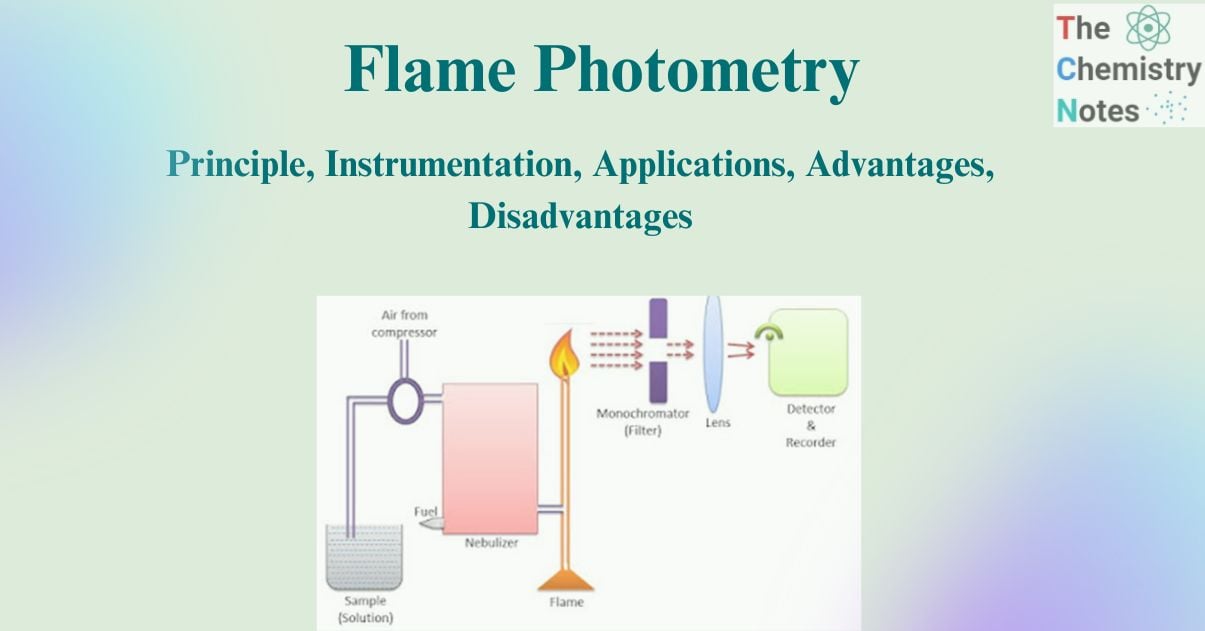
Instrumentation of Flame Photometry
The diagram to illustrate the instrumentation of flame photometry is given below:
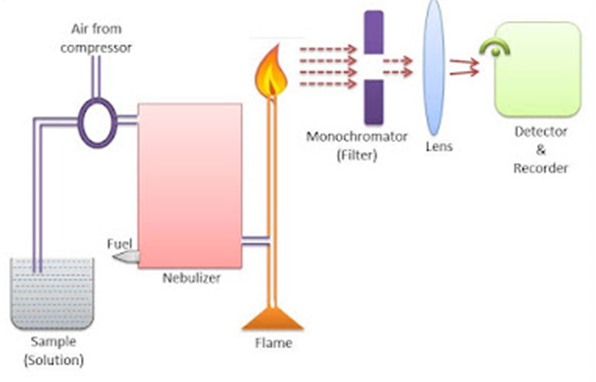
Fig: Instrumentation of Flame Photometry
image source: https://namrataheda.blogspot.com/2016/05/spectrophotometry-flame-photometry.html
The components which are required for flame photometry are given below:
Source of flame
A burner serves as a source of flame. It can be kept in a stable form and at a stable temperature. As an oxidant, flame photometry uses a number of fuels, primarily air, oxygen, or nitrous oxide (N2O). The temperature of the flame is affected by the fuel-oxidant ratio.
Nebulizer and mixing chamber
It helps to transport the homogeneous solution of the substance into the flame at a steady rate.
Optical system (optical filter) or Monochromator
In flame photometry, both the wavelength and the intensity of the radiation emitted by the elements must be monitored. As a result, a filter or monochromator should be utilized. The optical system is made up of three parts: a convex mirror, a lens, and a filter. Convex mirror aids in the transmission and focus of light emitted by the atoms. The convex lens aids in focusing light on a point known as the slit. Mirror reflections flow through the slit and reach the filters. This will separate the wavelength to be measured from any other unwanted emissions. As a result, it functions as an interference type color filter.
Detector
The detector’s primary function is to detect the emitted light. The intensity of the radiation emitted by the flame is measured by converting the emitted light into an electrical signal.
Amplifier
The amplifier is used in order to amplify the signals from the detector which is then received by the readout.
Readout Device
It reads the quantitative data and information regarding alkali or alkaline earth metals.
The various processes in the flame are discussed below:
1. Desolvation: The flame dehydrates the metal particles in the flame, causing the solvent to evaporate.
2. Vapourisation: this involves the vaporization of salt samples.
3. Atomization: The process by which metal ions in a solvent are reduced to metal atoms by the heat of the flame.
4. Excitation: process: The electrostatic force of attraction between the atom’s electrons and nucleus aids in the absorption of a specific amount of energy. The atoms then go to the excited energy state.
5. Emission process: Because the higher energy state is unstable, atoms emit energy in the form of radiation of a certain wavelength, which is recorded by the photodetector.
Interference in Flame Photometry
The intensity of the radiation does not precisely represent the concentration of the sample since other elements in the sample may influence the concentration of the specific element. As a result, the interference develops. Interferences in flame photometry are classified into three types:
Spectral interference
It occurs when the emission lines of two elements cannot be resolved or when it emerges from the flame’s background. They are either excessively close together, overlap, or occur as a result of a high salt concentration in the sample.
Ionic interference
Some metal atoms, such as sodium, may be ionized by a high-temperature flame. The Na+ ion has its own emission spectrum with frequencies that differ from those of the Na atom’s atomic spectrum.
Chemical interference
Chemical interferences result from the reactions of various interferents with the analyte.
• Interference between cations and anions: –
The presence of certain anions in a solution, such as oxalate, phosphate, and sulfate, can change the intensity of radiation emitted by an element. For example, – calcium + phosphate ion creates a stable compound, Ca3(PO4)2, which does not easily break down, resulting in the formation of fewer atoms.
• Cation-cation interference:
These interactions are neither spectral nor ionic in nature; for example, aluminum interacts with calcium and magnesium.
Advantages of Flame Photometry
Some of the advantages of flame photometry are as follows:
i. It is a quick, appropriate, and sensitive analysis;
ii. it is a simple and low-cost method of analysis
iii. it is employed in the qualitative and quantitative measurement of alkali and earth metal ions.
iv. The very low concentration of metal ions in the supplied sample can also be determined.
Disadvantages of Flame Photometry
Some of the limitations of flame photometry are discussed below:
i. It can only analyze liquid samples, and sample preparation can be time-consuming in some circumstances.
ii. A reference solution with known molarities is required to determine the ion concentration that will correspond to the emission spectra.
iii. It is not suitable for determining the metals directly.
iv. This method does not provide information on the molecular structure of the metal ion present in the sample.
v. Due to the presence of interferences, this approach cannot correctly determine the concentration of metal ions.
vi. The exact concentration of the metal ion in the solution cannot be determined.
vii. It cannot detect and determine the presence of inert gases directly,
viii. obtaining accurate results for ions with larger concentrations is challenging.
ix. Due to their non-radiative properties, certain elements, including carbon, hydrogen, and halides, cannot be detected.
Applications of Flame Photometry
Flame photometry is widely used in clinical chemistry, food and agriculture, environmental analysis, pharmaceutical industries, and so on. Some of its major applications are as follows:
- Soft drinks, fruit juices, and alcoholic beverages are also analyzed by flame photometry to determine the concentration of various metals and elements.
- It helps to determine the amount of the metals present in the sample of serum, urine, and other body fluids such as sodium, potassium, calcium, and lithium.
- It helps to estimate the amount of sodium and potassium in the glass.
- It helps to measure the amount of calcium and potassium in the soil, plant materials, food, and beverages.
- It helps to detect the presence of any specific element in the given sample.
- The concentrations of the sodium and potassium ions present in the human body can be determined by diluting and aspirating blood serum samples into the flame.
Limitations of flame Photometry
In spite of various applicability, flame photometry has some limitations, they are:
i. Changes in light emission as a result of changing flame temperature.
ii. The flame temperature must be precisely controlled.
iii. It is difficult to eliminate interference from other elements.
iv. Heavy and transition metals have a large number of absorption and emission lines, and their spectra are complicated.
v. Inadequate selectivity.
vi. Variations in viscosity between standards and samples.
Differences between Flame Photometry (Flame Emission Spectroscopy) and Atomic Absorption Spectroscopy
The differences between the flame photometry and atomic absorption spectroscopy are as follows:
| Flame Photometry (Flame Atomic Emission Spectroscopy) | Atomic Absorption Spectroscopy |
| It involves the emission phenomenon. | It involves the absorption phenomenon. |
| Radiation emitted by the excited atoms is proportional to the concentration. | Radiation absorbed during the excitation of the atoms is proportional to the concentration. |
| It has greater spectral interferences. | It has lesser spectral interferences. |
| It is mainly used for the analysis of alkali and alkaline earth metals. | It is used to analyse several metals like Al, Ag, Cu, Hg, Cd, etc. |
| It is lesser sensitive than atomic absorption spectroscopy. | It is more sensitive than flame atomic emission spectroscopy. |
Advantages of Atomic absorption spectroscopy over Flame Photometry
i. AAS shows higher sensitivity.
ii. It has high applicability.
iii. It has a smaller flame effect.
iv. AAS has less interference from other cations.
References
- Vogel’s Textbook of Quantitative Chemical Analysis, 6th Edition, 2008.
- https://www.kharagpurcollege.ac.in/studyMaterial/0422Flame-photometry-DSE2-for-SemV-12-02-2021.pdf
- H. H. Willard, L. L. Merritt, J. R. Dean, and F. A. Settle, Instrumental Methods of Analysis (7th Edition), CBS Publishers and Distributions, India, 1986.
- https://www.sciencedirect.com/topics/medicine-and-dentistry/flame-photometry.
- D.A. Skoog, F.J. Holler and T.A. Nieman, Principle of Instrumental Analysis, 5th Edition
