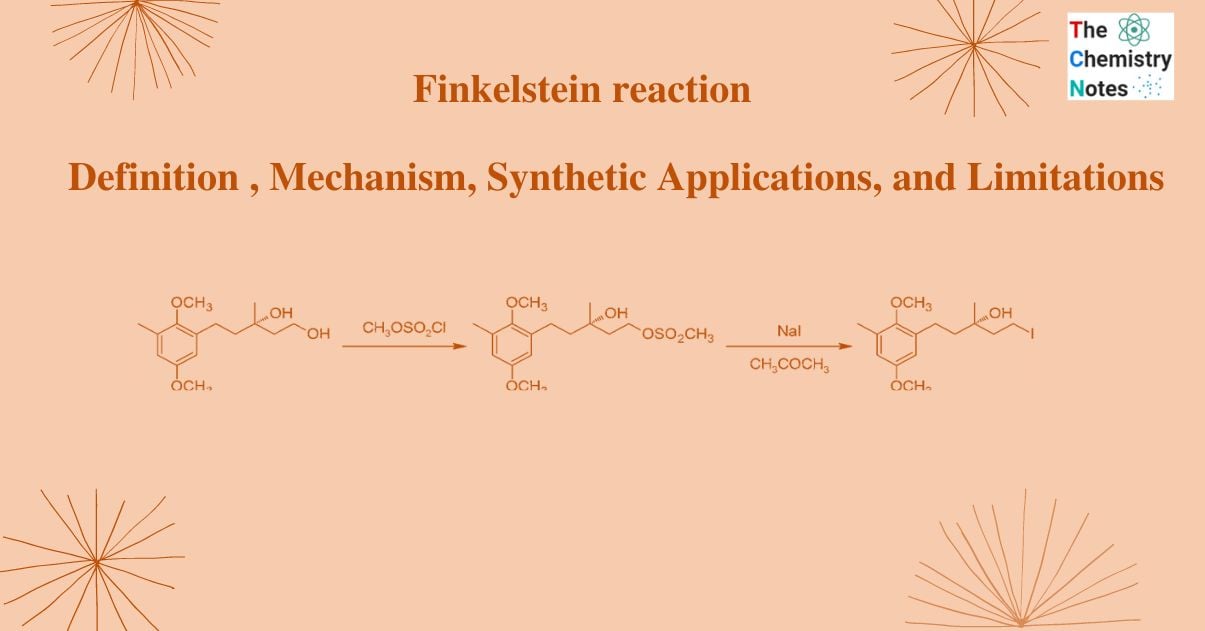
Finkelstein reaction is an organic process in which an alkyl halide reacts with a metal halide salt to form another alkyl halide.
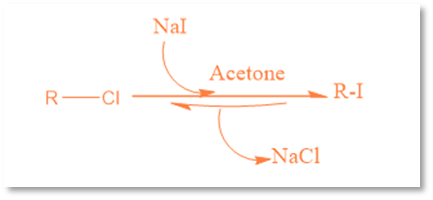
This reaction’s name is given after the German chemist Hans Finkelstein. It is a nucleophilic bimolecular substitution reaction in which one halogen atom is exchanged for another. Although it is an equilibrium reaction, it can be accelerated by taking advantage of the varying solubilities of halide salts or by utilizing a significant amount of the halide salt. By taking use of the newly created metal halide salt’s solubility in acetone, this equilibrium process is driven forward. Acetone does not dissolve sodium chloride or sodium bromide, but Acetone can dissolve sodium iodide. The reaction functions well with primary halides, but it performs best with allyl benzyl and -carbonyl halides.
For example: the conversion of methyl bromide to methyl iodide
CH3Br (acetone)+Nal (acetone)→CH3I (acetone)+NaBr (s)
Interesting Science Videos
Reaction mechanism of Finkelstein reaction
This reaction’s mechanism is a straightforward, one-step bimolecular nucleophilic substitution reaction (SN2), which proceeds with an inversion of stereochemistry.
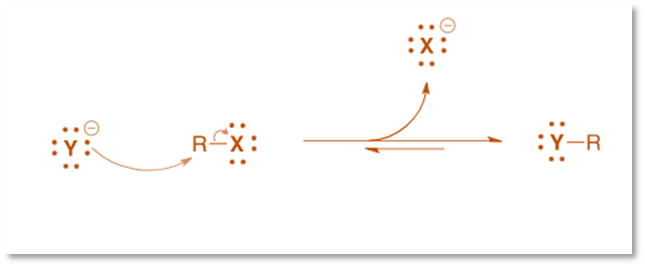
The reaction depends on the following factors:
- Nucleophilicity
- Nature of group
- Carbon-halogen bond
- Alkyl Halide reactivity
Aromatic Finkelstein reaction
Iodide cannot easily replace aromatic chlorides and bromides, however, this can occur under the right catalytic conditions. Copper(I) iodide in conjunction with diamine ligands. Besides CuI, other potential catalysts include nickel bromide and tri-n-butyl phosphine.
Uses of Finkelstein reaction
- It is utilized in the analysis of alkyl halides because the ease with which they undergo the reaction varies substantially among alkyl halides.
- This reaction is used to produce alkyl iodides efficiently.
- By first converting the alcohol to a sulfonate ester, it is also used to convert alcohols to alkyl halides.
- To identify the class of an unknown halide, a qualitative test using NaI in acetone can be utilized.
- It is also used to produce chrysochlamic acid.
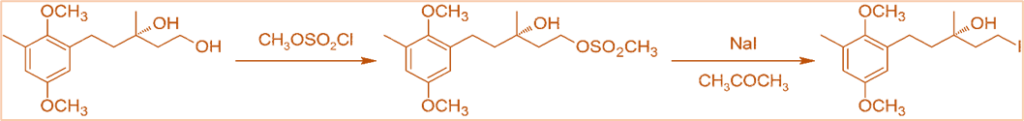
Limitations of Finkelstein reaction
Secondary halides have a much lower reactivity. Halides of vinyl, aryl, and tertiary alkyl do not react in this reaction.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
