Ferrocene, also known as bis(η5-cyclopentadienyl)iron or (C5H5)2Fe, is a finely powdered substance with an orange hue. It is widely regarded as one of the most extensively researched metallocenes. As aforementioned, the complex adheres to the 18-electron rule and exhibits exceptional stability. The Cp− ligands of the compound can be readily modified to incorporate various functional groups. Ferrocene derivatives that have been functionalized are presently utilized as biosensors in equipment designed for measuring blood glucose levels. Additionally, they are the subject of extensive investigation as prospective agents for combating cancer.
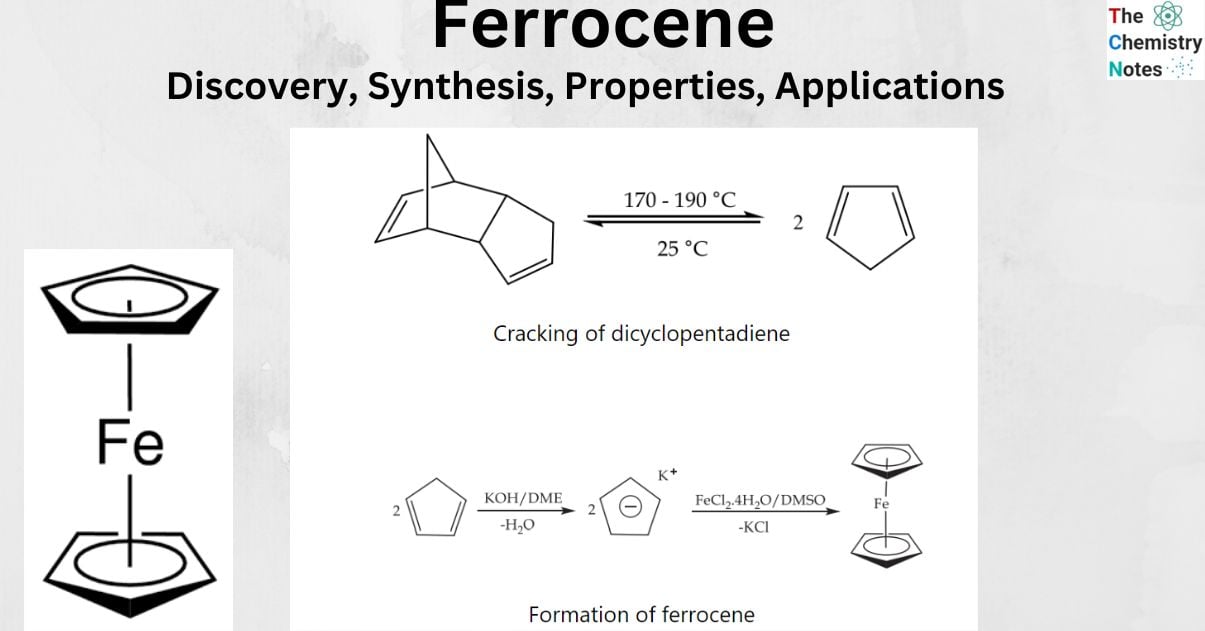
The chemical formula for ferrocene is represented as Fe(C5H5)2, and it is classified as an organometallic compound. A core iron atom sits at the heart of the molecule, which is a complicated structure made up of two cyclopentadienyl rings linked together. It is a solid that has an orange color, has an odor that is similar to camphor, and sublimes as the room temperature elevates. It is soluble in the majority of organic solvents.
![Ferrocene [Fe(η-C5H5)2]](https://scienceinfo.com/wp-content/uploads/2023/06/image-240.png)
Discovery of Ferrocene
Ferrocene was found in 1951 using the same random process that led to the discovery of the bulk of chemical compounds. German chemist, Peter L. Pauson and American chemist, Thomas J. Kealy made an effort to produce fulvalene ((C5H4)2) by oxidative dimerization cyclopentadiene (C5H6).
To accomplish this, they subjected cyclopentadienyl magnesium bromide (C5H5BrMg) and ferric chloride to a reaction in the hopes of oxidatively coupling the diene. Despite this, they were successful in obtaining a light orange powder that exhibited “remarkable stability.” The aromatic nature of the negatively charged cyclopentadienyl was thought to be responsible for this stability; however, these cyclopentadienyl were unable to recognize the sandwich structure of the η5 (pentahapto) molecule.
2 C5H5MgBr + FeCl2 → Fe(C5H5)2 + MgBr2 + MgCl2
Based on the substance’s reactivity, an American organic chemist, Robert Burns Woodward and English chemist, Geoffrey Wilkinson were able to deduce its structure. Ernst Otto Fischer arrived at the same conclusion regarding the sandwich structure independently and began to synthesize further metallocenes, such as nickelocene and cobaltocene.
Some of these compounds are examples of metallocenes. Both nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography were able to validate the structure of ferrocene. Because of its unique “sandwich” structure, it sparked a surge of interest in compounds involving d-block metals and hydrocarbons, which in turn led to the formation of the now-thriving field of organometallic chemistry.
German chemist, Ernst Otto Fischer and English chemist, Sir Geoffrey Wilkinson shared a Nobel Prize in 1973 for their work on organometallic chemistry. Ferrocene is more efficiently produced via the reaction of sodium cyclopentadienyl with anhydrous ferrous chloride in ethereal solvents.
Electronic Structure of Ferrocene
The +2 oxidation state of the central iron atom in ferrocene has been acknowledged, which can be demonstrated through the utilization of Mössbauer spectroscopy.
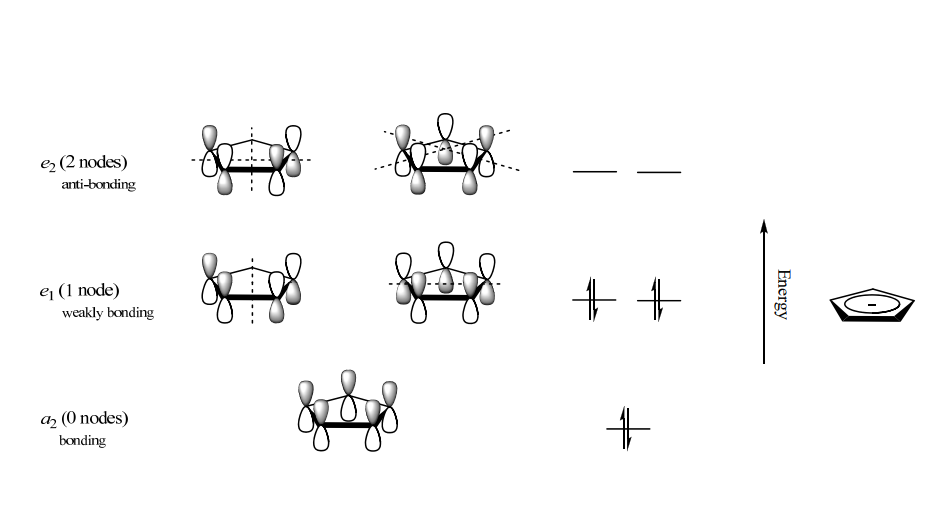
A single negative charge is assigned to each cyclopentadienyl ring, resulting in the occupation of a π orbital by an extra electron. This brings the number of π-electrons on each ring to six, thereby conferring aromaticity upon them.
The complex achieves an 18-electron, inert gas electron configuration by sharing twelve electrons (six from each ring) with the metal through covalent bonding. This is combined with the six d-electrons of Fe2+. The stability of ferrocene is enhanced by this configuration.
- Ferrocene two cyclopentadienyl (Cp) rings can adopt two distinct conformations: eclipsed (D5h) or staggered (D5d).
- The rotational energy pertaining to the Fe-Cp axis is of negligible magnitude, approximately 4 kJmol-1. Consequently, the fundamental configurations of ferrocene may exhibit either of these conformations.
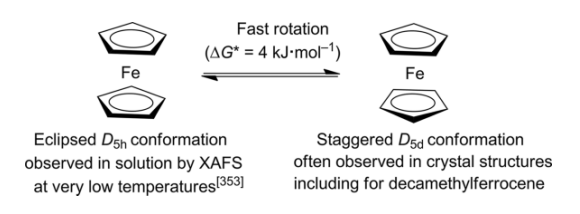
- The electronic states of D5h and D5d symmetries exhibit minimal disparity. Nonetheless, the electronic structure of ferrocene is explicated using the D5d point group representations due to their facilitation of symmetry matching between ligand molecular orbitals and metal atomic orbitals.
- The formation of metal-ligand bonds in ferrocene is primarily attributed to the interactions between the Fe orbitals and the π-orbitals of the Cp ligand. Assuming D5d symmetry, whereby a center of symmetry exists in the ferrocene molecule through the Fe atom, there will be the presence of centro-symmetric (g) and anti-symmetric (u) combinations.
- The molecular orbitals of five p-orbitals on a planar Cp ring with D5h symmetry can be generated through their combination.
Synthesis of Ferrocene
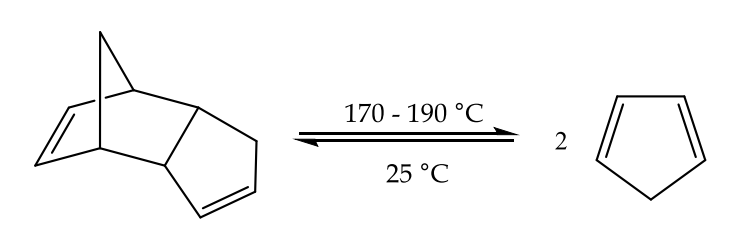
At ambient temperature, monomeric cyclopentadiene (C5H6) undergoes rapid dimerization through a Diels-Alder reaction, resulting in the formation of dicyclopentadiene. The process of obtaining cyclopentadiene through fractional distillation involves the thermal decomposition (cracking) of commercial dicyclopentadiene.
Industrial Synthesis
Ferrocene is made in factories by mixing iron (II) ethoxide with cyclopentadiene. Making iron (II) ethoxide involves electrochemically oxidizing metallic iron in anhydrous ethanol. Since the reaction between iron (II) ethoxide and cyclopentadiene produces ethanol as a byproduct, the ethanol effectively catalyzes the overall reaction, with the net reaction being:
Fe + 2 C5H6 → H2 + Fe (C5H5)2
Through Grignard Reagent
Multiple sources almost simultaneously produced ferrocene for the first time. Ferrocene was synthesized by Pauson and Kealy through the reaction of iron (III) chloride with a Grignard reagent, specifically cyclopentadienyl magnesium bromide. The process involves the suspension of iron (III) chloride in anhydrous diethyl ether and its subsequent addition to the Grignard reagent.
The given chemical process involves a redox reaction that results in the formation of the cyclopentadienyl radical and iron (II) ions. The production of dihydro fulvalene occurs through a process of radical-radical recombination. Meanwhile, the reaction between iron (II) and the Grignard reagent results in the formation of ferrocene. The desired outcome of Kealy and Pauson, which is the oxidation of dihydro fulvalene to fulvalene using iron (III), does not take place.
Gas-Metal Reaction
Miller et al. performed the other early synthesis of ferrocene. They did this by reacting metallic iron directly with gas-phase cyclopentadiene at a high temperature. This was the first known method of producing ferrocene.There was also a method that used iron pentacarbonyl that was disclosed.
Fe(CO)5 + 2 C5H6 (g) → Fe(C5H5)2 + 5 CO (g) + H2 (g)
Through Alkali Cyclopentadienide
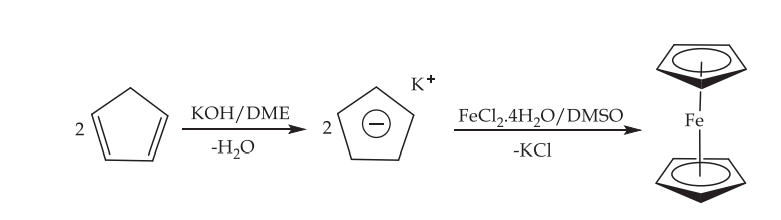
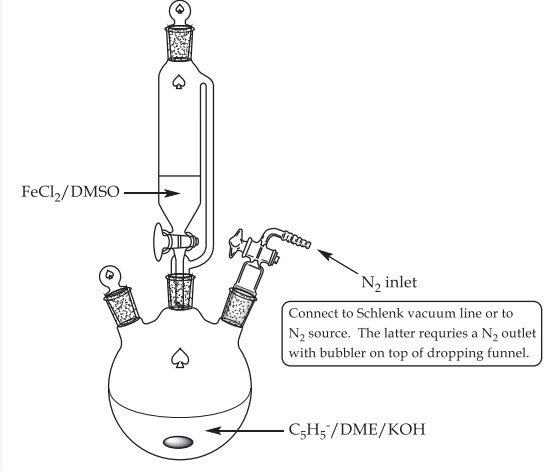
The initial transmetalation process is usually altered in preparative procedures that are generally more successful in this trend. In these processes, either sodium cyclopentadienide, which can be procured in commercial quantities, or freshly cracked cyclopentadiene, which has first been deprotonated with potassium hydroxide and then reacted with anhydrous iron (II) chloride in ethereal solvents, is used. Both of these processes are carried out in the same manner.
Both of these techniques are going to be broken down in the following paragraphs.
It is well-known that Pauson and Keely’s original Grignard method has undergone significant revisions and enhancements in the recent past.
Using sodium cyclopentadienide:
2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
Using freshly-cracked cyclopentadiene:
FeCl2·4H2O + 2 C5H6 + 2 KOH → Fe(C5H5)2 + 2 KCl + 6 H2O
Using an iron (II) salt with a Grignard reagent:
2 C5H5MgBr + FeCl2 → Fe(C5H5)2 + 2 MgBrCl
Even some amine bases, such as diethylamine, can be utilized for deprotonation; however, the rate at which the reaction occurs is significantly slower when utilizing amine bases as compared to stronger bases.
2 C5H6 + 2 (CH3CH2)2NH + FeCl2 → Fe(C5H5)2 + 2 (CH3CH2)2NH2Cl
The preparation of ferrocene via other metallocenes, like manganocene, can also be accomplished using the process of direct transmetalation:
FeCl2 + Mn(C5H5)2 → MnCl2 + Fe(C5H5)2
Physical Properties of Ferrocene
The orange solid known as ferrocene is stable in air but easily sublimes in a vacuum, particularly when heated. Ferrocene is soluble in typical organic solvents such as benzene, but it does not dissolve in water. This is the behavior that would be predicted for a symmetric and uncharged species.
At temperatures near 100 degrees Celsius, ferrocene begins to sublimate noticeably.
| Compound Formula | C10H10Fe |
|---|---|
| Molecular Weight | 186.03 |
| Appearance | Orange crystalline solid |
| Melting Point | 172-174 °C |
| Boiling Point | 249 °C |
| Density | 1.1-1.49 g/cm3 |
| Solubility in H2O | Insoluble |
| Absorption | λmax 358 nm |
| Exact Mass | 186.013188 |
| Monoisotopic Mass | 186.013188 |
Chemical Properties of Ferrocene
The Friedel-Crafts Acylation of Ferrocene
The Friedel-Crafts acylation is a type of electrophilic aromatic substitution reaction that facilitates the incorporation of an acyl group onto an aromatic ring. The acyl cation, which serves as the electrophile, is frequently associated with a Lewis acid catalyst, such as aluminum chloride. For the reaction to occur, it is imperative that the aromatic ring system exhibits a high degree of electron richness, thereby precluding the presence of any electron-withdrawing groups.
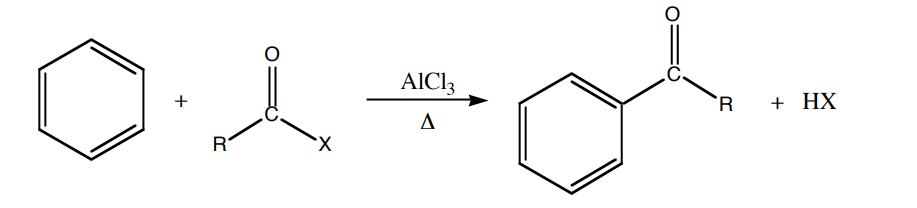
The metallocene compound, commonly referred to as the “sandwich” compound, was fortuitously discovered in 1951 and Ferrocene serves as a prominent illustration of this class of compounds.
The cyclopentadienyl rings present in ferrocene exhibit a significant degree of electron density, rendering them aromatic in nature. Consequently, these rings are susceptible to a range of reactions that are analogous to those observed in benzene. It is noteworthy that a majority of metallocenes exhibit higher reactivity towards electrophilic reagents in comparison to benzene, thereby implying greater accessibility of electrons. The Friedel-Crafts acylation of benzene necessitates the utilization of aluminum chloride as a catalyst. However, ferrocene can undergo acylation with acetic anhydride under less severe conditions with the aid of phosphoric acid as the catalyst.
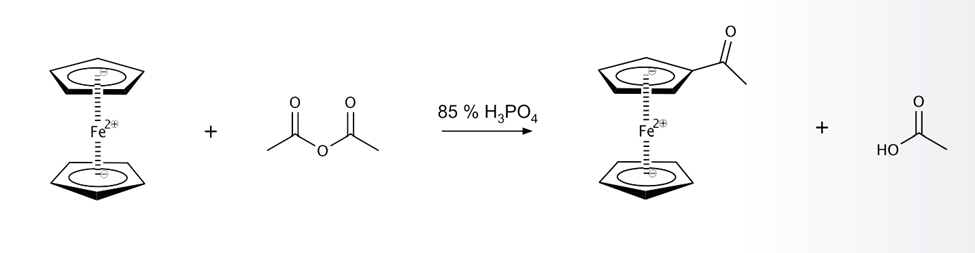
Mechanism:
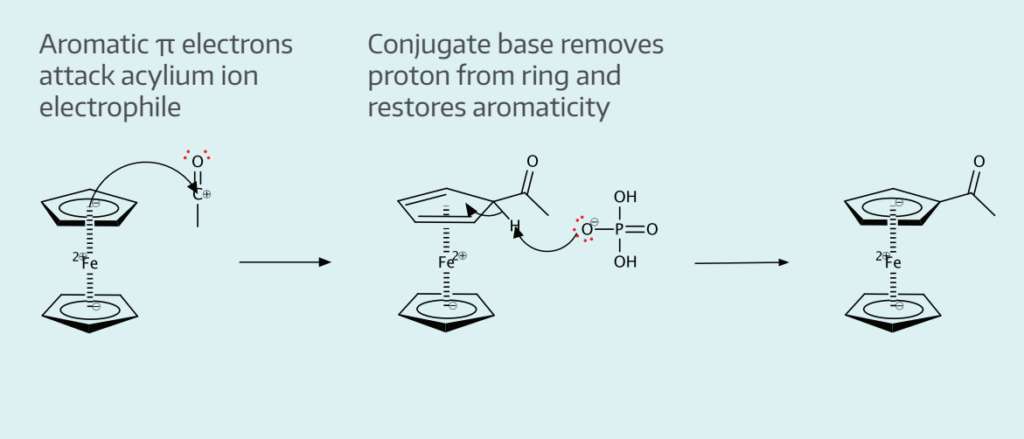
The reaction between acetic anhydride and phosphoric acid yields the acylium ion electrophile. Acetylferrocene is formed as a result of the electrophilic attack by the nucleophilic ferrocene.
- Protonation of acetic anhydride with phosphoric acid
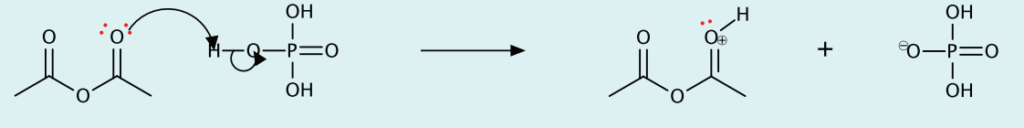
- Cleavage of C-O bond to form acetic acid and acylium ion
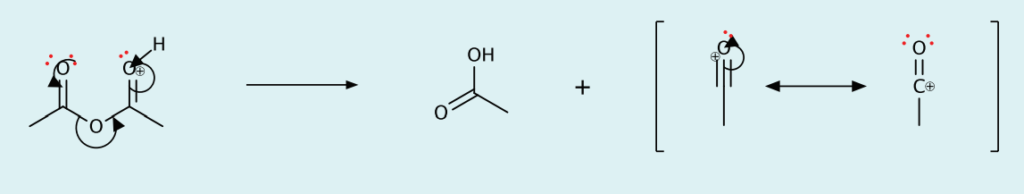
- Electrophilic aromatic substitution of H on ferrocene with acylium ion
Applications of Ferrocene and its derivatives
Ferrocene exhibits limited practical applications. Nevertheless, established synthetic techniques facilitate the production of innumerable derivatives, thereby broadening the scope of potential applications.
Fuel Additives
Fuel additives are chemical compounds that are added to fuel in order to improve its performance or characteristics. Ferrocene and its derivatives are utilized as antiknock agents in petrol engine fuel, replacing the previously employed tetraethyl lead due to their superior safety profile. Ferrocene has been observed to decrease the formation of particulate matter in engines powered by diesel fuel.
Medical
Ferrocene and its derivatives have been subject to rigorous examination as potential agents for combating cancer. This scrutiny persists to this day. At the outset, a variety of ferrocenium salts were evaluated for their cytotoxic efficacy. Although the precise mechanism of action remains uncertain, various targets such as DNA, cell membrane, and enzymes have been suggested. It is postulated that Ferrocenium salts have the capacity to produce hydroxyl radicals within physiological parameters. These agents have the potential to cause harm to the DNA structure, potentially through the process of DNA oxidation. Moreover, it is postulated that the cellular membrane could potentially serve as a target.
Studies have demonstrated that a counter-ion is a critical factor in determining a substance’s cytotoxic efficacy and aqueous solubility. Ferrocenium salts’ picrate and trichloroacetate derivativesexhibit notable aqueous solubility and significant cytotoxicity. As a component of this study, attaching ferrocene to polymers was effectively executed to enhance their aqueous solubility and, consequently, their cytotoxic potential.
Certain ferrocenium salts have demonstrated potential as anticancer agents, and a ferrocenyl derivative of tamoxifen has been developed as an experimental drug. The proposed concept is that tamoxifen will selectively attach to the estrogen binding sites, leading to a cytotoxic impact.
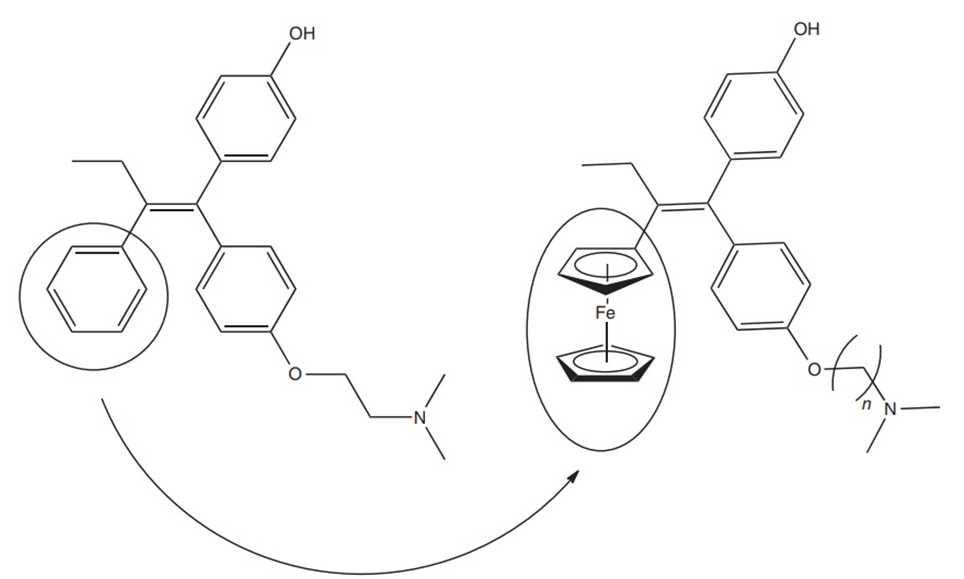
Ferrocene Polymers for Cathodic Battery Materials
The incorporation of ferrocene-containing side chains into polymers has been found to exhibit advantageous characteristics such as air stability, rapid electrochemical kinetics, and consistent voltage plateaux. These properties make them promising candidates for the advancement of cathodic battery materials. The electrical insulation property of these polymers imposes a constraint on their application as energy storage materials, necessitating their use in thin layers to ensure rapid charging and discharging kinetics. The introduction of conductive polymers has the potential to enhance electronic conductivity.
Material Chemistry
The field of study that investigates the properties, structure, and synthesis of materials is known as materials chemistry. Due to its high sublimation capacity, Ferrocene has been identified as a viable option for the deposition of specific types of fullerenes, particularly carbon nanotubes. The versatility of organic reactions in modifying ferrocenes enables the synthesis of vinyl ferrocene. Vinyl ferrocene can be synthesized through a Wittig reaction involving an aldehyde, a phosphonium salt, and sodium hydroxide. The vinyl ferrocene has the potential to undergo polymerization, resulting in the formation of a polymer that can be regarded as a ferrocenyl derivative of polystyrene, wherein the phenyl moieties are substituted with ferrocenyl groups.
Ferrocene finds application as a nano-scale template in the production of ultra-high molecular weight polyethylene’s elongated fibers, which are utilized in the fabrication of advanced bullet-resistant vest material.
In Supramolecular Ensembles, Liquid Crystals, and Nonlinear Optical Materials
The incorporation of ferrocene units into macrocycles, such as cryptands and calixarenes, has been observed. This has been particularly studied by Beer’s group for its potential use in anion redox sensing. This group has synthesized a significant quantity of endo receptors containing ferrocene and utilized them for anion sensing.
In recent times, there has been a pursuit of ion-pair recognition and sensing in aqueous solutions.
The selectivity of ferrocene-appended interlocked structures, specifically rotaxanes, towards chloride ions as opposed to more basic oxoanions. This was determined through the use of 1H NMR spectroscopy and electrochemistry. Notably, the selectivity observed in these structures differed significantly from that of their acyclic counterparts. The research group led by Tarraga and Molina has developed a chemosensor utilizing a ferrocene-based imidazo phenanthroline dyad. This sensor has demonstrated high efficacy in detecting aqueous hydrogen pyrophosphate as well as the organic anions ADP and ATP through three distinct channels.
Ligand scaffold
The application of chiral ferrocenyl phosphines as ligands in transition-metal catalyzed reactions is a common practice. Certain compounds have been utilized in industrial settings for the purpose of synthesizing pharmaceuticals and agrochemicals. The diphosphine compound, 1,1′-Bis(diphenylphosphino)ferrocene (dppf), comprises a ferrocene moiety and is a highly useful ligand in palladium coupling reactions.
Ferrocene-Containing Stars and Their Electrostatic Effects in Electron-Transfer Processes
The utilization of the hexsubstituted benzene framework is highly advantageous in the fabrication of hex-ferrocene stars and the evaluation of their noteworthy redox, electrostatic, and electrochemical characteristics. The inclusion of ferrocene units in stars is exemplified by the synthesis of highly congested hexaferrocenylbenzene.
In Nanomedicine
The field of medicine has shown significant interest in organometallics, particularly in the study of the ferrocene/ferricenium redox couple, which has been subject to extensive research. The substance is presently employed as a redox mediator in the amperometric identification of glucose in blood, a crucial aspect of blood analysis for individuals with diabetes.
The oxidation of the reduced form of glucose oxidase enzyme (GODred) is facilitated by Ferricenium, which is generated at the anode. Subsequently, the oxidized form of the enzyme (GODox) is produced, which in turn catalyzes the oxidation of the glucose substrate to gluconolactone.
Ferrocene-chalcogen-triazole-sugar conjugates, nitroaryl ferrocenes and PEGylated ferrocene radiosensitizer compounds are investigated for their potential interactions with biological membranes, their ability to bind to tubulin, and their modification of phospholipids.Ferrocene-iminosugar hybrids, ferrocene-N-heterocyclic carbene-gold(I) complexes with a focus on antioxidant pathways, and strategies related to dendrimers have been investigated.
Ferrocene: Hazard Information
- Harmful
- Highly flammable
- Dangerous for the environment
- Harmful if swallowed
- Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
Prevention and Safety
- It is recommended to utilize electrical, ventilating, and lighting equipment that is designed to withstand and prevent explosions.
- It is advised to refrain from consuming food or beverages or smoking while utilizing this smoking activities while utilizing this particular product.
- It is recommended to refrain from releasing substances or materials into the surrounding environment.
- It is recommended to utilize protective gloves, protective clothing, eye protection, and face protection to ensure safety.
- Response
- In the event of ingestion, it is imperative to promptly contact a medical professional or poison control center.
References
- https://www.aiinmr.com/wp-content/uploads/2019/10/Friedel-Crafts-Acetylation-of-Ferrocene.pdf
- Werner H (June 2012). “At least 60 years of ferrocene: the discovery and rediscovery of the sandwich complexes”. Angewandte Chemie. 51 (25): 6052–6058. doi:10.1002/anie.201201598.
- https://www.magritek.com/wp-content/uploads/2015/03/Lab-Manual-Synthesis-and-Reactions-of-Ferrocene-web.pdf
- Pavia, D., L.; Lampman, G., M.; Kriz, G., S.; Engel, R., G. Introduction to Organic Laboratory Techniques: A Small Scale Approach; Thomson Brooks/Coles, 2005.
- Woollins, J., D. Inorganic Experiments; Wiley-VCH Verlag GmbH & Co. KGaA, 2010.
- Shriver, D., F.; Drezdzon, M., A. The Manipulation of Air-Sensitive Compounds; John Wiley & Sons, Inc., 1986
- https://www.americanelements.com/ferrocene-102-54-5
- https://www.azom.com/
- Knox GR, Pauson PL, Willison D (1992). “Ferrocene derivatives. 27. Ferrocenyldimethylphosphine”. Organometallics. 11 (8): 2930–2933. doi:10.1021/om00044a038.
- https://www.pharmacy180.com/article/ferrocene-1420/
- https://chemistnotes.com/inorganic/ferrocene-properties-structure-uses/
- https://chemistryeurope.onlinelibrary.wiley.com/doi/full/10.1002/ejic.201600983
