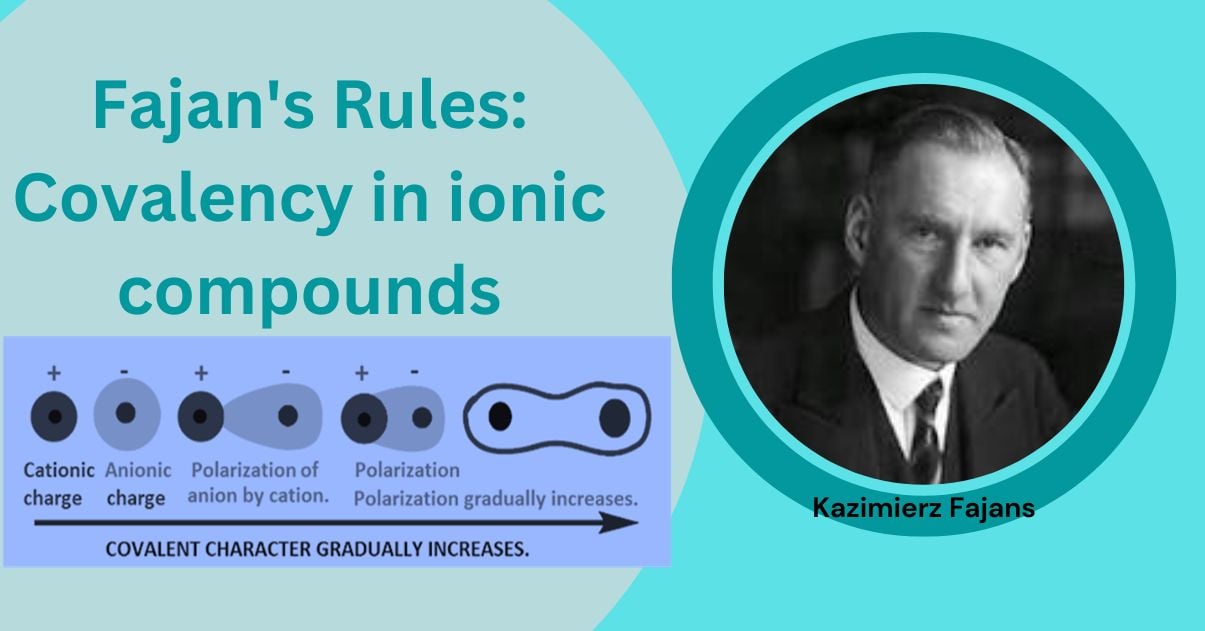
Fajan’s rules can be used to determine the tendency of ionic compounds to acquire covalent character as a result of polarization. K. Fajan studied the factors which introduced the covalent character in the ionic compounds and purposed a set of rules which are known as Fajan’s rules. Based on the relative charges and sizes of the cation and anion, Kazimierz Fajans developed a set of rules in 1923 that can be used to determine whether a chemical bond is likely to be mostly ionic or covalent.
Despite the fact that a bond in a molecule like X+Y- may be entirely ionic, it will always have some covalent properties. When two ions with opposite charges (X+ and Y-) come into contact, the cation attracts electrons in the anion’s outermost shell but repels the positively charged nucleus. Because of this, the anion is polarized, deformed, or distorted. An ionic bond forms when the degree of polarization is relatively low, whereas a covalent bond develops when the degree of polarization is high. An anion’s tendency to become polarized by the cation is known as its polarizability, while a cation’s capacity to deform an anion is known as its polarization power.
Interesting Science Videos
Factors favoring covalence character in ionic compounds
According to Fajan, the following factors favor the covalence character in ionic compounds.
A. Smaller cations favor covalency
Smaller ions can significantly polarize the anion due to their extremely strong polarizing power. A cation’s ability to polarize becomes stronger as its size gets smaller. As a result, the covalent nature of a molecule increases (i.e., ionic character decreases).
Example:
Polarizing power : Li+ > Na+ > K+ > Rb+ > Cs+
As the radius of the cation increases →
Therefore, Polarizing power decreases →
B. Large anion favors the covalency
Large anion has a high tendency to get distorted (i.e., high polarizability). Polarization of anion increases in anionic radius. The high polarizability of anion develops the covalency.
Polarizability: I–> Br– > Cl– > F–
As the radius of the anion decreases →
Therefore, Polarizability decreases →
C. High charge on cation and anion favors covalency:
A cation with a high positive charge has high polarizing power and an anion with a high negative charge have high polarizability. These factors favor the development of covalency.
Example: Polarizing power of cations = Na + < Mg 2+ < Al3+
Polarizability of anion = F– < O2- < N3-
D. Cations with pseudo-noble gas configuration (18 es ) on valence shell favor covalency
When compared to compounds with noble gas configuration (8 es) on the valence shell, cations with pseudo-noble gas configuration (18 es) on the valence have a higher polarizing power.
For instance, the valence shells of Na+, K+, and Rb+ exhibit noble gas configurations (8 es). They are therefore mainly form ionic molecules. The Cu+, Ag+, and Au+ on the other hand, have a pseudo-noble gas configuration on their valence shell and mostly form covalent compounds.
Applications of Fajan’s rules
1. This rule determines the ionic or covalent nature of the compound.
2. It is used to determine the melting point and solubility of ionic compounds.
For example, although K + and Ag + ions are almost the same the melting point of KCl is higher than that of the AgCl. This can be explained in terms of electronic configuration. The electronic configuration of K+ is [Ar], while that of Ag+ is [Kr] 4d10. As the Ag+ exhibit a pseudo-nobel configuration, it would be more polarizing.
Percentage of ionic character
Every ionic molecule is predicted to have at least a small amount of covalent character based on Fajan’s rules. Dipole moments can be used to determine a compound’s percentage of ionic nature.
References
- https://protonstalk.com/chemical-bonding/fajans-rule/
- http://www.adichemistry.com/general/chemicalbond/fajan/fajans-rules.html
- https://byjus.com/jee/fajans-rule/
- https://www.vedantu.com/iit-jee/fajans-rule
- http://www.lscollege.ac.in/sites/default/files/e-content/FAJANS_RULE.pdf
