Exothermic reactions are chemical reactions that generate heat. The term exothermic is derived from the Greek words Exo, which means outside, and thermic, which means heat. As a result, exothermic heat is heat that moves outside. Exothermic reactions are significant in forensic science, notably in the examination of fires and explosions. Endothermic reactions occur when a chemical reaction demands heat (rather than creating it) and results in the cooling of the surroundings. Exothermic reactions is often released as heat, making the surrounding environment hotter. An exothermic process is exemplified by hand warmers

What is an Exothermic Reaction?
An exothermic reaction is a chemical process that involves the release of energy in the form of heat or light. For example, a lot of heat is created when carbon burns in oxygen to make carbon dioxide.
The concept that atomic bonds break and energy is released when the reactants in an exothermic reaction prepare to transform during a chemical reaction is included in the definition of an exothermic reaction.
The total energy of the products in an exothermic reaction is less than the total energy introduced into the system by the reactants. Heat is one of the results of an exothermic reaction, as shown by the equation:
A + B –> AB + Heat
Exothermic reactions can occur spontaneously and increase the system’s unpredictability or entropy (ΔS > 0). They are distinguished by a drop in enthalpy (ΔH < 0) and a negative heat flow (heat is lost to the environment). Exothermic reactions in the lab generate heat and may even be explosive.
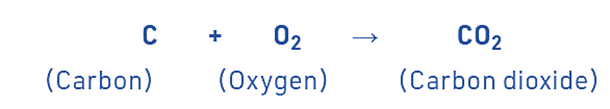
A lot of heat is created when carbon burns in oxygen to make carbon dioxide.
Chemical Processes that are Exothermic
- Fuel combustion, including wood, coal, and oil/petroleum
- Thermite reactivity
- Alkali metals and other highly electropositive metals react with water.
- Condensation of rain from water vapor
- mixing water with strong bases or acids
- The chemical interaction of acids and bases
- Dehydration of carbohydrates by sulfuric acid
- The cement and concrete setting
- Certain polymerization processes, such as epoxy resin setting
- Nuclear fusion in nuclear weapons and stellar cores (to iron)
- Heavy element nuclear fission
- The interaction of zinc with hydrochloric acid
- Respiration (breaking down of glucose to release energy in cells) (breaking down of glucose to release energy in cells)
Exothermic Reactions and Energy Changes
The term exothermic implies “heating up.” When an exothermic process progresses, energy, frequently in the form of heat, is released. When the energy created in an exothermic reaction is released as heat, the temperature rises. As a result, the products will almost certainly be hotter than the reactants. An exothermic reaction has the following general equation:
Reactants → Products + Energy
When a process absorbs or emits heat under constant pressure, enthalpy change happens. The enthalpy change (ΔH) may be calculated as the difference between the enthalpy of the products and the enthalpy of the reactants. The energy diagram of an exothermic chemical process shows that the reactants have more energy than the products. As a result, ΔH is negative (ΔH < 0), and the process is exothermic.
A reaction profile is more informative than an energy level diagram in describing how the energy of the molecules changes during the reaction.
The activation energy, which is the lowest energy required by particles when they collide for a reaction to occur, is included in a reaction profile. The activation energy is represented by a hump in the line, which begins when the energy of the reactants equals the energy difference between the top of the ‘hump’ and the reactant
The difference in energy between the reactants and products is the overall change in energy in a reaction.
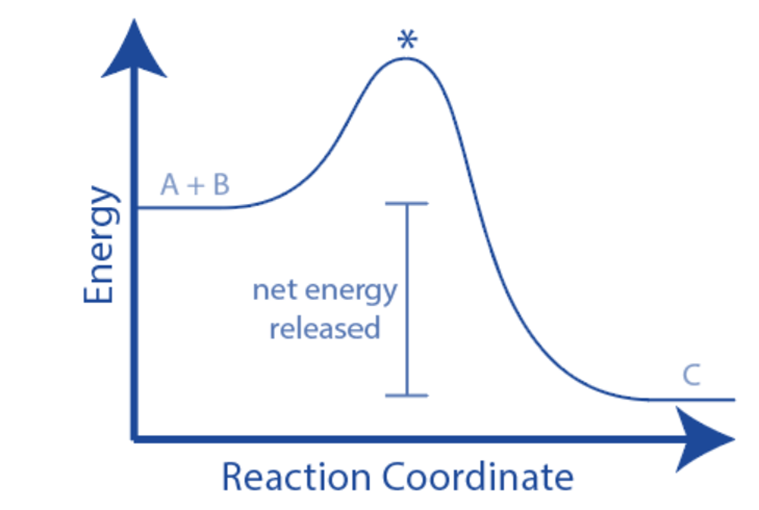
The energy of the product is lower than the energy of the reactants, for an exothermic process (as thermal energy has been transferred to the surroundings)
Exothermic processes transmit thermal energy to the surroundings, raising the temperature of the surroundings. This energy is transferred from the chemical energy storage of the chemical system to the surroundings, causing the system’s energy to decline – indicating a negative energy shift. The entire transfer occurs from the system to the environment.
Difference Between Exothermic and Exergonic Reaction
Exergonic and exothermic processes both produce energy. The released energy in the exothermic reaction is simply referred to as energy and energy released in an exergonic reaction, has a special name called Gibbs energy or Gibbs free energy.
Energy of reactants in exothermic reaction is greater than that of products whereas in exergonic energy reactants is greater than that of the products.
The energy of the reaction system reduces in comparison to that of the surroundings, causing the surroundings to get hotter. It has nothing to do with whether the reactants become hot or cold in an exergonic reaction. It has a deeper chemical significance; it refers to the spontaneity of the reaction; hence, it always signifies that a reaction is possible, i.e. the reaction will always occur. In summary, an exergonic reaction is one that occurs spontaneously.
Exothermic Reaction with Chemical Equations
Combustion
Combustion is the process through which a substance burns in the presence of oxygen, emitting heat and light. These reactions provide a lot of energy in the form of heat, but they also produce certain byproducts like smoke. These are some examples of combustion:
- Lighting a match
- the lighting of fireworks
- Burning the coals
- Setting on a gas oven
- a piece of paper being burned
Natural gas is mostly composed of methane. Carbon dioxide and water vapor are created when natural gas is burnt in the presence of oxygen in the air. A large amount of thermal energy is also generated. Here’s an example of how this may be expressed:

Respiration
Respiration is regarded as an exothermic reaction. Glucose (C6H12O6) from carbohydrates combines with oxygen (O2) in cells, releasing carbon dioxide (CO2) and water (H2O) and providing energy to the human body. The burning of glucose is another example of a combustion process, as shown by the following reaction:

Formation of Snow
Ice crystals generated from water vapor in clouds in the atmosphere are what precipitate snow. As the temperature falls below 0 ⁰C or 32 ⁰F, the water transforms from a liquid to a solid (freezing).
H2O (l) → H2O (s) + heat
Condensation Reaction
When the temperature drops, the water vapor in the clouds condenses and falls as rain.
H2O (g) → H2O (l) + heat
Ion Pair Formation
Ions interact with water while they are gaseous, resulting in hydrated ions. This is an exothermic reaction. The greater the ion charge, the smaller the ion radius. As a result, the enthalpy of ion hydration increases, resulting in a concentrated exothermic reaction.
As we all know, heat is required to break a chemical bond. Hydrogen chloride, for example, is a diatomic molecule composed of a hydrogen atom H and a chlorine atom Cl joined by a covalent single bond.
Reaction of Strong Acid and Water
HCl (Hydrogen chlorides) is a powerful acid, and when it is mixed with water, the hydrogen ion combines with the water to produce a more complex reaction. Because hydrogen ions are so small in size, a tremendous quantity of energy is concentrated in a comparatively small region. At this point, it attracts polar water molecules.
Some other Exothermic Reaction in Laboratories
Exothermic reactions in laboratories, industry, and power plants are illustrated here.
- Sodium chloride (NaCl) is produced by combining sodium (Na) and chlorine (Cl).
2 Na (s) + Cl2 (g) → 2 NaCl (s) + heat
- Calcium oxide (CaO) reacts violently with water (H2O) to form calcium hydroxide (Ca(OH)2), often known as slacked lime.
CaO (s) + H2O (l) → Ca(OH)2 (aq.) + heat
- When calcium chloride (CaCl2) combines with water (H2O), hydrochloric acid (HCl) and calcium oxide are formed (CaO).
CaCl2 (s) + H2O (l) → CaO (s) + 2 HCl (aq.) + heat
- Ammonia synthesis: Haber’s technique is used in ammonia synthesis. In this reaction, nitrogen (N2) from the air mixes with hydrogen (H2) primarily generated from natural gas (methane) to form ammonia (NH3).
N2 (g) + 3 H2 (g) → 2 NH3 (g) + heat
References
- Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015). Principle of Modern Chemistry, Brooks Cole. p. 617. ISBN 978-1305079113
- Laidler, K. J. (1996). “A glossary of terms used in chemical kinetics, including reaction dynamics (IUPAC Recommendations 1996)”. Pure and Applied Chemistry
- “Exothermic & Endothermic Reactions | Energy Foundations for High School Chemistry”. highschoolenergy.acs.org. Retrieved 2021-04-11.
- https://www.chemistrylearner.com/chemical-reactions/exothermic-reaction
- Helmenstine, Anne Marie, Ph.D. “Examples of Endothermic Reactions.” ThoughtCo, Sep. 7, 2021, thoughtco.com/endothermic-reaction-examples-608179.
- https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/17.3/primary/lesson/exothermic-reactions-ms-ps/
- Austin, Patrick (January 1996). “Tritium: The environmental, health, budgetary, and strategic effects of the Department of Energy’s decision to produce tritium”. Institute for Energy and Environmental Research. Retrieved 2010-09-15.
