Ethanol Definition
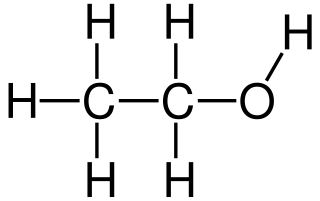
Ethanol or ethyl alcohol is the primary alcohol that is formed by the substitution of a hydrogen atom in the alkane, ethane by a hydroxyl group.
- It is an organic compound composed of carbon, hydrogen, and oxygen with the molecular formula C2H5OH. The formula can be written as CH3-CH2-OH.
- It is a highly volatile, flammable liquid with a faint characteristic odor. It is often used as a psychoactive substance or a recreational drug.
- Ethanol can be produced naturally by the fermentation of carbohydrates by yeast and other microorganisms. It can also be produced artificially via petrochemical processes like ethylene hydration.
- Ethanol is one of the most common alcohols that is recognized by many as it is the active ingredient in alcoholic drinks.
- It is also considered an alternative fuel source and is used as a chemical solvent in the synthesis of various other organic compounds.
- Besides being used in alcoholic beverages, different concentrations of ethyl alcohol can be used as antiseptic or disinfectant.
- Ethanol is also used as rocket fuel and is used in lightweight rocket-powered racing aircraft.
- Ethanol is less polar than methanol and thus is less acidic than water. The presence of two methyl groups allows the distribution of electrons between different atoms, which decreases the polarity of the molecule.
Methanol Definition
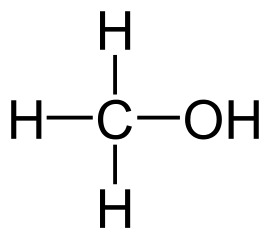
Methanol is the primary alcohol that is formed by the substation of a hydrogen atom in the alkane methane by a hydroxyl group.
- Methanol is a highly volatile and flammable liquid with a characteristic alcoholic odor that is similar to that of ethanol.
- Methanol was traditionally called wood alcohol as it was produced by the distillation of wood. Nowadays, it is primarily produced by the hydrogenation of carbon monoxide.
- The molecular formula of methanol is CH3OH, where the methyl group is attached to a polar hydroxyl group.
- Methanol is the simplest aliphatic alcohol which is often used as an amphiprotic solvent, fuel as well as human metabolite.
- The vapors of methanol can mix completely with water; however, these are slightly heavier than air and can travel a particular distance to a source of ignition.
- Methanol is a highly toxic alcohol that can cause permanent blindness by the consumption of about 10 ml.
- The toxicity of methanol can occur via two means; methanol can cause fatal effects on the central nervous system in the form of depressants, and it can also be metabolized into formic acids, which is toxic as it inhibits mitochondrial cytochrome c oxidase.
- Methanol poisoning has been observed in the form of outbreaks as a result of contamination of drinking alcohol.
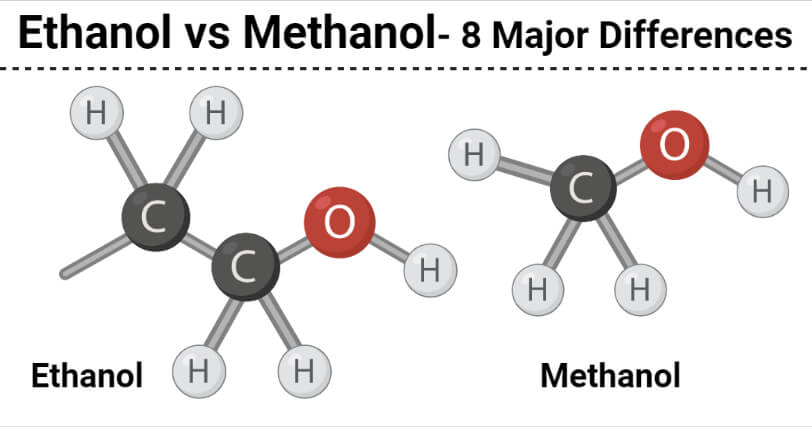
8 Key Differences (Ethanol vs Methanol)
| Characteristics | Ethanol | Methanol |
| Definition | Ethanol or ethyl alcohol is a primary alcohol that is formed by the substitution of a hydrogen atom in the alkane, ethane by the hydroxyl group. | Methanol is a primary alcohol that is formed by the substation of a hydrogen atom in the alkane methane by a hydroxyl group. |
| Chemical formula | The chemical formula of ethanol is C2H5OH. | The chemical formula of methanol is CH3OH. |
| Acidity | Ethanol is a weaker acid. | Methanol has a stronger acid. |
| Use | Ethanol is produced by the fermentation of sugar in the presence of yeasts. | Methanol is synthetically produced by the catalysis of carbon monoxide, carbon dioxide, and hydrogen. |
| Nature | Ethanol is used as an active ingredient in alcoholic beverages. | Methanol is highly toxic and not suitable for consumption. |
| Molar mass | Ethanol has a higher molar mass. | Methanol has a lower molar mass. |
| Boiling point | Ethanol has a higher boiling point. | Methanol has a lower boiling point. |
| Chemical Nature | Ethanol is a renewable liquid. | Methanol is a non-renewable liquid. |
References and Sources
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 887, Methanol” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Methanol. Accessed 16 March 2021.
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 702, Ethanol” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Ethanol. Accessed 16 March 2021.
- 2% – https://www.uchemchemicals.com/organic-intermediates/organic-reagent-and-other-heterocycles/methyl-alcohol-cas-67-56-1.html
- 2% – https://www.differencebetween.com/difference-between-ethanol-and-vs-methanol/
- 2% – https://en.wikipedia.org/wiki/Anhydrous_ethanol
- 1% – https://www.vedantu.com/chemistry/methanol
- 1% – https://www.osti.gov/biblio/5260923-catalytic-hydrogenation-carbon-monoxide
- 1% – https://www.hazmattool.com/info.php?a=Methanol&b=UN1230&c=3
- 1% – https://www.foodstandards.gov.au/publications/documents/TR2.pdf
- 1% – https://www.engineeringtoolbox.com/ethanol-ethyl-alcohol-properties-C2H6O-d_2027.html
- 1% – https://www.differencebetween.com/difference-between-ethanol-and-bioethanol/
- 1% – https://www.chemicalsafetyfacts.org/ethanol/
- 1% – https://petrochemistry.eu/interactive-flowchart/
- 1% – https://pediaa.com/difference-between-ethyl-alcohol-and-ethanol/
- 1% – https://pediaa.com/difference-between-ethanol-and-methanol/
- 1% – https://conservative.ly/toxic-methanol-that-causes-blindness-found-in-hand-sanitizers-fda-warns
- 1% – https://brainly.com/question/11006244
- 1% – https://blablawriting.com/the-use-of-ethanol-as-an-alternative-fuel-source-essay
- 1% – http://chemistry.elmhurst.edu/vchembook/213organicfcgp.html
- <1% – https://www.researchgate.net/publication/339092710_Methanol_outbreak_A_Malaysian_tertiary_hospital_experience
