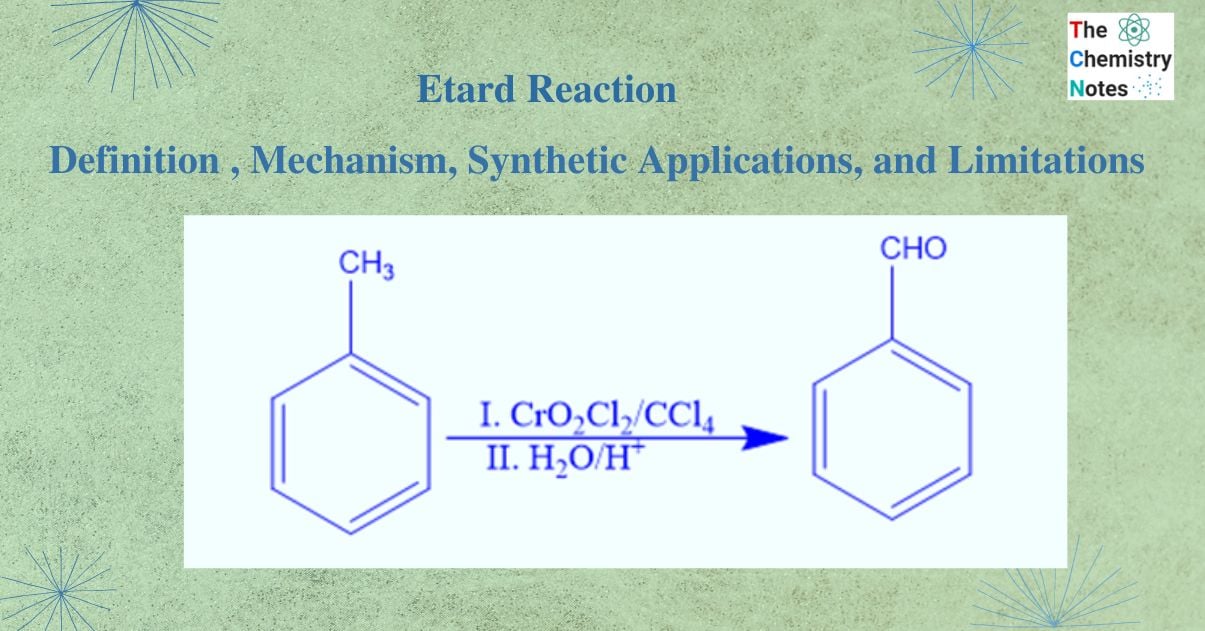
Etard reaction is a chemical reaction that involves the direct oxidation of an aromatic or heterocyclic bound methyl group to an aldehyde using chromyl chloride. It is named after French Scientist Alexandre Leon Etard. In this reaction, chromyl chloride serves as the oxidizing agent. In this reaction aromatic methyl groups or methyl groups bound to heterocyclic rings are oxidized to the corresponding aldehyde. Using this reaction, toluene is most commonly oxidized to benzaldehyde. The usefulness of this reaction is that it stops methyl group oxidation at the aldehyde group without proceeding to the carboxylic acid.
Étard complex is produced as a result of an ene reaction with chromyl chloride. In order to stop further oxidation to a carboxylic acid, the Étard complex is then broken down by a [2,3] sigmatropic rearrangement. Saturated aqueous sodium sulphite provides reducing conditions for the breakdown of the Etard complex. Dichloromethane and carbon disulfide are typical solvents for the reaction.
The initial clark red color of the solution changes to a dark brown to black during the exothermic addition of chromyl chloride to the toluene as the complex slowly precipitates. These findings also suggest that the aromatic and chromyl chloride first form an π- or σ-complex, followed by the formation of the true solid complex.
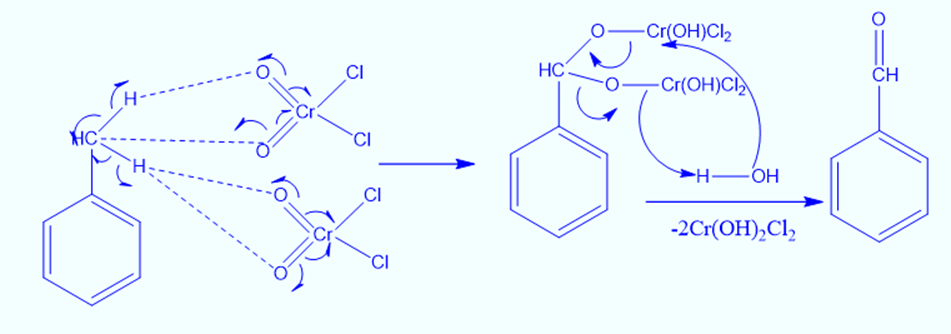
Reaction mechanism of Etard oxidation
In the Etard reaction, chromyl chloride, a weak oxidizing agent, first reacts with toluene in the presence of carbon tetrachloride, a non-polar solvent. Homolytic cleavage of the chromyl chloride -bonds occurs during this reaction. In a similar manner, the methyl group’s C-H bonds are also broken down by homolytic cleavage. As a result, the Etard complex or chromyl complex is formed.
The etard complex is now hydrolyzed, which causes the removal of two Cr(OH)2Cl2 molecules and forms benzaldehyde (Aldehyde).
Applications of Etard oxidation
- It is a useful method for the conversion of toluene to aldehyde.
- Benzaldehyde acts as a precursor for a range of substances, such as pharmaceuticals, dyes, and perfumes.
- The food industry also uses benzaldehyde to give food products an almond flavor.
Limitations of Etard oxidation
Most frequently, the Étard reaction is used as a straightforward way to turn toluene into benzaldehyde. Rearrangements make it challenging to produce specific aldehyde products from reagents other than toluene. The reaction also produces more stable carboxylic acids if strong oxidizing agents are used.
Suggested video
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
